Abstract
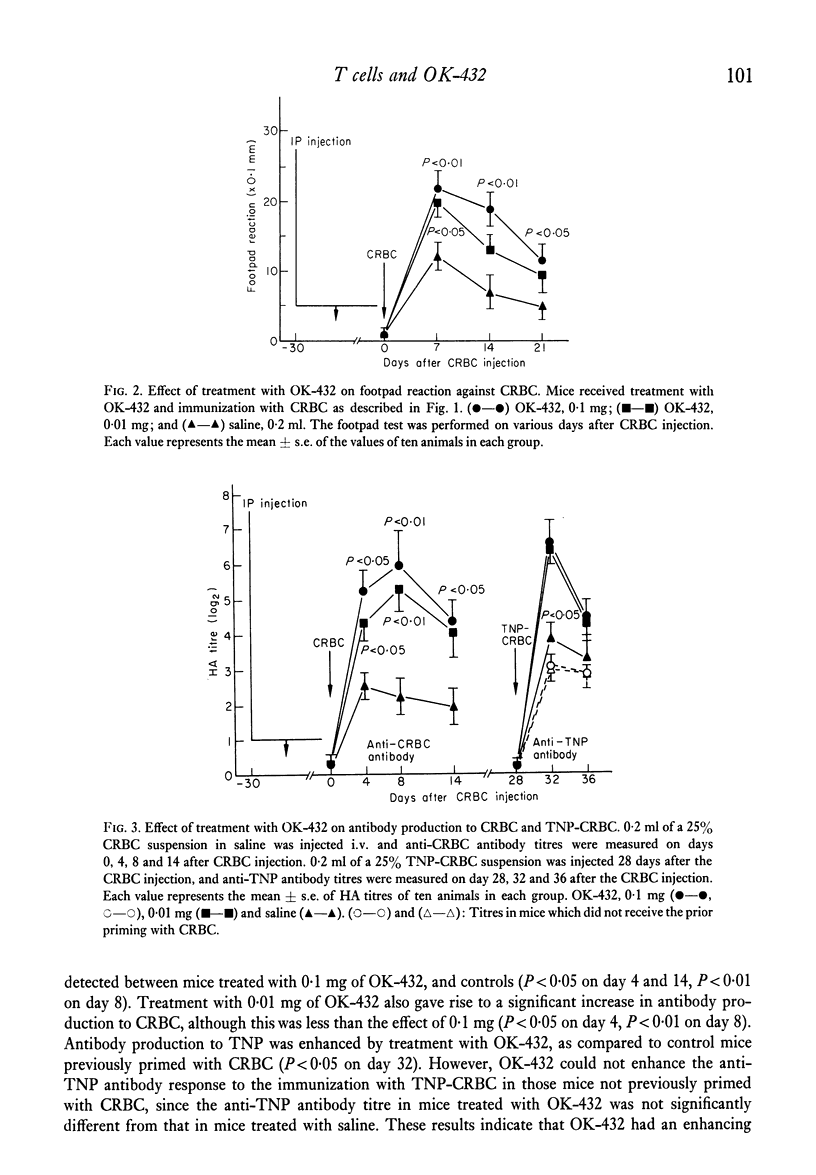
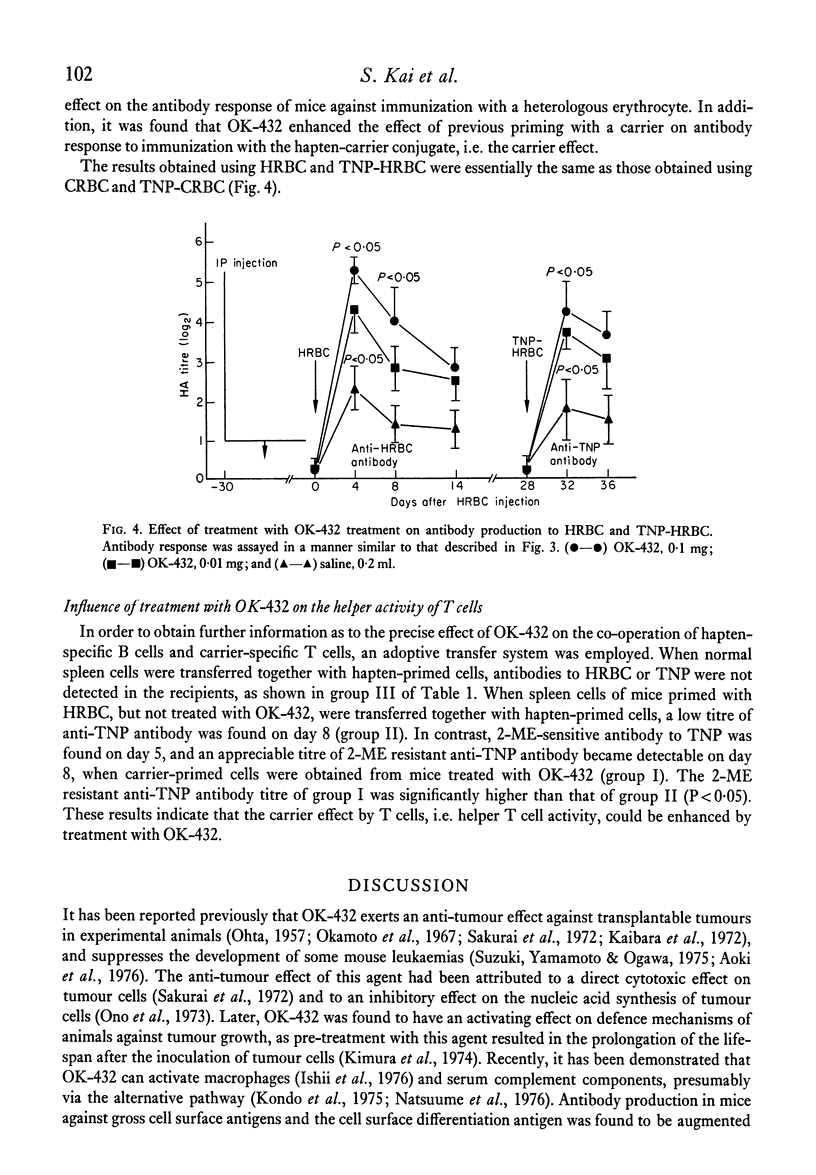
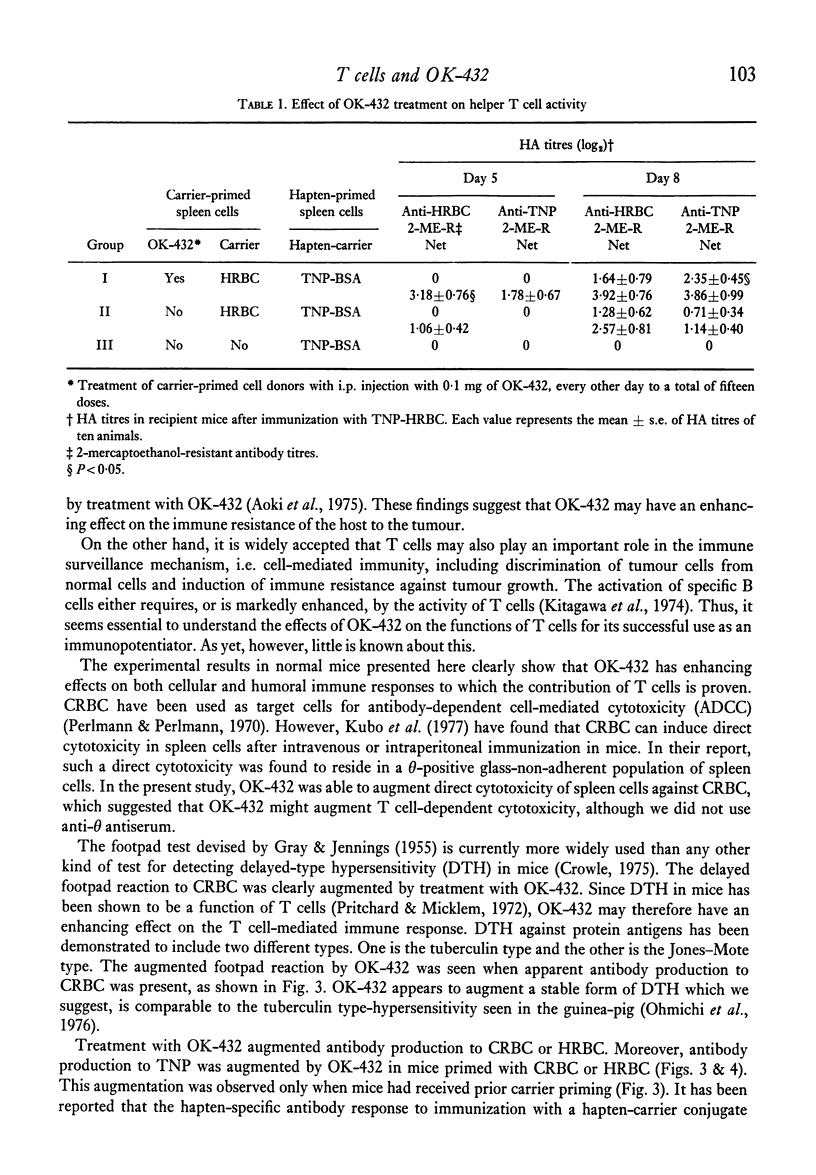
The effects of the anti-tumour agent OK-432 on the immune response to hamster erythrocytes (HRBC) and nucleated chicken erythrocytes (CRBC) were studied in inbred SL mice. Mice were treated repeatedly with OK-432 before immunization with erythrocytes in saline. The cytotoxicity of CRBC-primed spleen cells, as demonstrated by 51Cr release from labelled CRBC, was markedly increased by treatment with OD-432. The delayed footpad reaction to CRBC was significantly augmented by treatment with OK-432. These results in mice indicate that OK-432 can enhance the cellular immune responses which require the contribution of T cells. Such an activation of T cells by OK-432 was observed in the humoral immune response to a trinitrophenyl group. Augmentation of anti-hapten antibody production, suggesting the enhancement of helper T cell activity by OK-432, was noticed after immunization with trinitrophenyl conjugated to erythrocytes. Furthermore, this enhancement of helper T cell activity by OK-432 was confirmed by utilizing an adoptive transfer system. These results support the possibility that T cell activation may be one of the important effects of OK-432 as an immunopotentiator.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Kvedar J. P., Hollis V. W., Jr, Bushar G. S. Streptococcus pyogenes preparation OK-432: immunoprophylactic and immunotherapeutic effects on the incidence of spontaneous leukemia in AKR mice. J Natl Cancer Inst. 1976 Mar;56(3):687–690. doi: 10.1093/jnci/56.3.687. [DOI] [PubMed] [Google Scholar]
- Askonas B. A., Roelants G. E. Macrophages bearing hapten-carrier molecules as foci inducers for T and B lymphocyte interaction. Eur J Immunol. 1974 Jan;4(1):1–4. doi: 10.1002/eji.1830040102. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Doria G., Agarossi G., Boraschi D., Antonietta M. Effect of carrier priming on antibody avidity in the in vivo and in vitro immune response. Immunology. 1977 Apr;32(4):539–548. [PMC free article] [PubMed] [Google Scholar]
- Erb P., Feldmann M. Role of macrophages in in vitro induction of T-helper cells. Nature. 1975 Mar 27;254(5498):352–354. doi: 10.1038/254352a0. [DOI] [PubMed] [Google Scholar]
- Freedman L. R., Cerottini J. C., Brunner K. T. In vivo studies of the role of cytotoxic T cells in tumor allograft immunity. J Immunol. 1972 Dec;109(6):1371–1378. [PubMed] [Google Scholar]
- GRAY D. F., JENNINGS P. A. Allergy in experimental mouse tuberculosis. Am Rev Tuberc. 1955 Aug;72(2):171–195. doi: 10.1164/artpd.1955.72.2.171. [DOI] [PubMed] [Google Scholar]
- Grant C. K., Evans R., Alexander P. Multiple effector roles of lymphocytes in allograft immunity. Cell Immunol. 1973 Jul;8(1):136–146. doi: 10.1016/0008-8749(73)90100-7. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Yamaoka H., Toh K., Kikuchi K. Inhibition of tumor growth in vivo and in vitro by macrophages from rats treated with a streptococcal preparation, OK-432. Gan. 1976 Feb;67(1):115–119. [PubMed] [Google Scholar]
- Kaibara N., Ikeda T., Hattori T., Inokuchi K. Effectiveness of treatment using a streptococcal preparation (PC-B-45) and mitomycin-C on transplanted mouse tumor. Gan. 1972 Jun;63(3):387–390. [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. Radioresistance of the enhancing effect of cells from carrier-immunized mice in an in vitro primary immune response. Proc Natl Acad Sci U S A. 1971 Apr;68(4):699–703. doi: 10.1073/pnas.68.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Onoshi T., Yasuhara S., Watanabe T., Sugiyama M., Hiraki K. Studies on the host-mediated action of the streptococcal preparation, OK-432, in cancer chemotherapy. Acta Med Okayama. 1974 Dec;28(6):423–431. [PubMed] [Google Scholar]
- Kitamura Y., Nomoto K., Torisu M., Takeya K. Effects of BCG (Bacillus Calmette-Guérin) vaccines on immune responses in mice. I. Possible effect of BCG on helper T cells. Jpn J Microbiol. 1976 Aug;20(4):303–308. doi: 10.1111/j.1348-0421.1976.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Kondo M., Ikezaki M., Imanishi H., Nishigaki I., Hosokawa K. Streptococcal preparation as an activator of host-mediated immune response: cellular immunity and alternate pathway of complement. Gan. 1975 Dec;66(6):675–678. [PubMed] [Google Scholar]
- Kubo C., Nomoto K., Sato M., Takeya K. Direct cytotoxicity against chicken erythrocytes in mice. I. Fundamental nature of T cell-mediated cytotoxicity. Immunology. 1977 Dec;33(6):895–905. [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- Ohmichi Y., Nomoto K., Yamada H., Takeya K. Relationships among differentiated T-cell subpopulations. I. Dissociated development of tuberculin type hypersensitivity, Jones-Mote type hypersensitivity and activation of helper function. Immunology. 1976 Jul;31(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Shoin S., Koshimura S., Shimizu R. Studies on the anticancer and streptolysin S-forming abilities of hemolytic streptococci. Jpn J Microbiol. 1967 Dec;11(4):323–326. doi: 10.1111/j.1348-0421.1967.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Ono T., Kurata S., Wakabayashi K., Sugawara Y., Saito M. Inhibitory effect of a streptococcal preparation (OK-432) on the nucleic acid synthesis in tumor cells in vitro. Gan. 1973 Feb;64(1):59–64. [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Immune responses in congenitally thymus-less mice. I. Absence of response to oxazolone. Clin Exp Immunol. 1972 Jan;10(1):151–161. [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Schirrmacher V., Nase S., Jerne N. K. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969 Jun 1;129(6):1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Amkraut A. A. Immunogenicity of trinitrophenyl-hemocyanin: production of primary and secondary anti-hapten precipitins. J Immunol. 1966 Sep;97(3):421–430. [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Sakai S., Ryoyama K., Koshimura S., Migita S. Studies on the properties of a streptococcal preparation OK-432 (NSC-B116209) as an immunopotentiator. I. Activation of serum complement components and peritoneal exudate cells by group A streptococcus. Jpn J Exp Med. 1976 Apr;46(2):123–133. [PubMed] [Google Scholar]
- Suzuki S., Yamamoto A., Ogawa H. Inhibitory effect of a streptococcal preparation (OK-432) on induction of splenomegaly by Friend leukemia virus. Gan. 1975 Aug;66(4):455–456. [PubMed] [Google Scholar]
- Torisu M., Fukawa M., Nishimura M., Harasaki H., Kai S., Tanaka J. Immunotherapy of cancer patients with Bacillus Calmette-Guérin: summary of four years of experience in Japan. Ann N Y Acad Sci. 1976;277(00):160–186. doi: 10.1111/j.1749-6632.1976.tb41696.x. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi S., Sakurai Y., Sato H., Akiba T SUZUKI S. Tumor-inhibitory effect of a streptococcal preparation (NSC-B116209). Cancer Chemother Rep. 1972 Feb;56(1):9–17. [PubMed] [Google Scholar]
- Unanue E. R., Katz D. H. Immunogenicity of macrophage-bound antigens: the requirement for hapten and carrier determinants to be on the same molecule for T and B lymphocyte collaboration. Eur J Immunol. 1973 Sep;3(9):559–563. doi: 10.1002/eji.1830030907. [DOI] [PubMed] [Google Scholar]


