Abstract
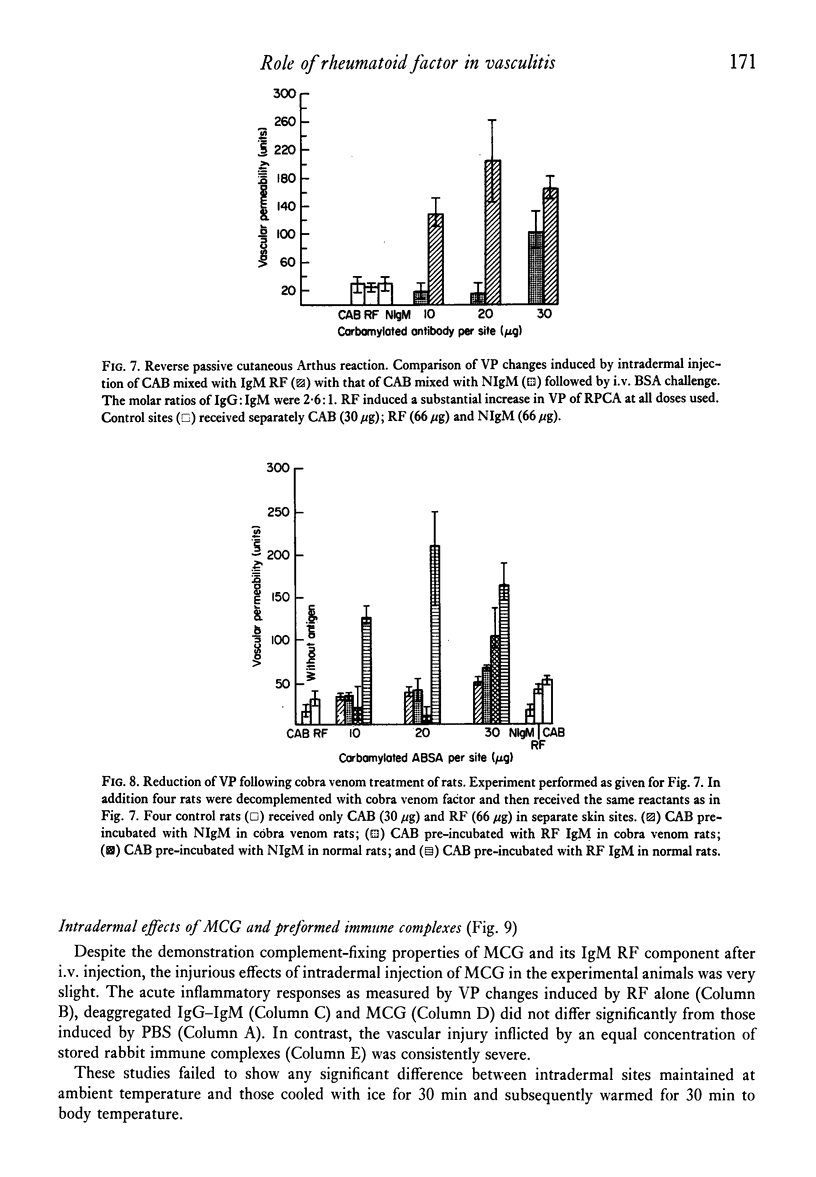
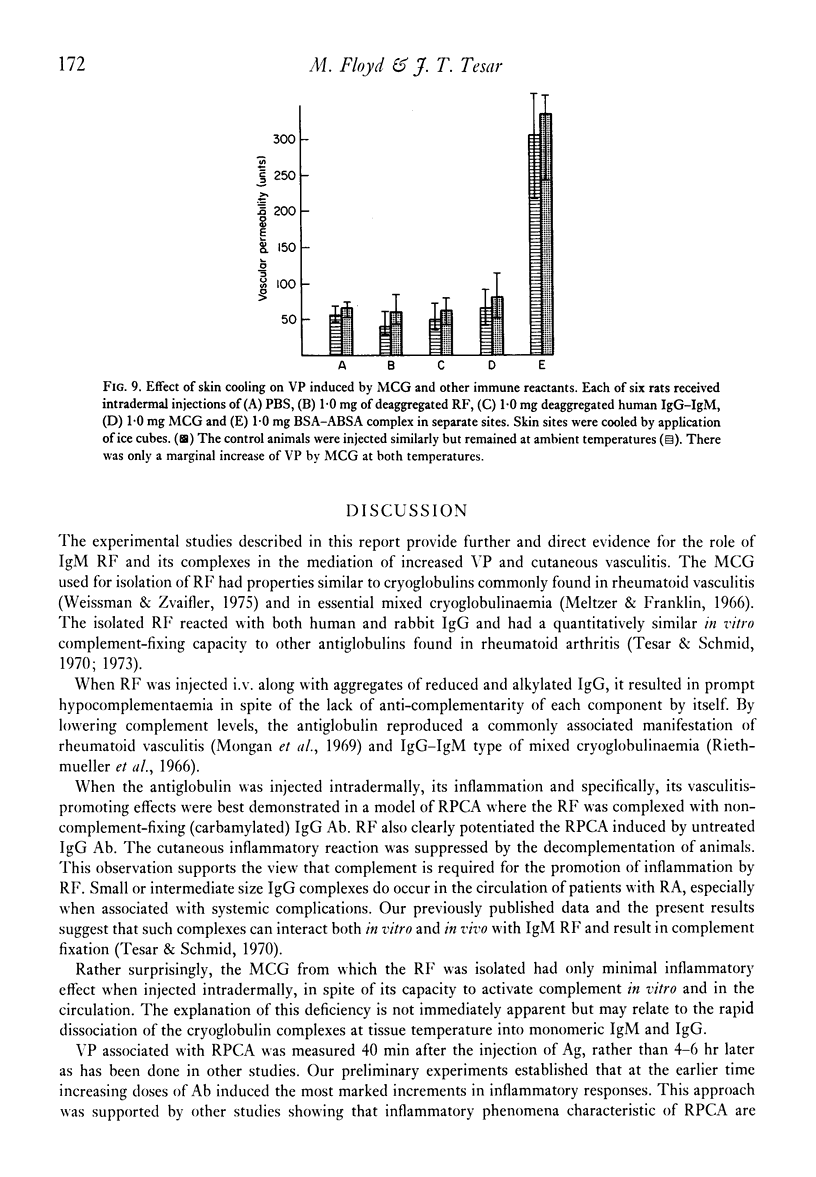
The effect of IgM rhematoid factor (RF) on reversepassive cutaneous Arthus reaction in rats was studied. The RF was obtained from the serum cryoglobulin of a patient with symptoms of purpura, arthralgia and digital gangrene. The cryoglobulins was of IgG-IgM type and when given i.v it induced a prompt hypocomplementaemia in experimental animals. The purified RF also induced low serum complement levels when injected i.v. along with complexes of non-complement-fixing, aggregated IgG. A reverse passive Arthus reaction was induced by intradermal injection of IgG anti-bovine serum albumin (BSA), followed by an i.v. dose of antigen (Ag). The cutaneous inflammatory reaction was aggravated by simultaneous administration of IgM RF intradermally, but not by IgM without antibody (Ab) properties. Intradermal injection of low concentrations of non-complement-fixing IgG anti-BSA, along with normal human IgM, followed by i.v. injection of BSA, resulted in a complete lack of cutaneous inflammation. At higher Ab concentrations there was only a mild inflammation. However, when IgM RF was substituted for normal IgM and injected with non-complement-fixing anti-BSA, an effective reverse passive cutaneous Arthus reaction and vasculitis was induced. The inflammatory response was greatly suppressed by decomplementation of animals by cobra venom factor. This study provides evidence favouring an inflammatory, complement-dependent role for RF in vasculitis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUM J., STASTNY P., ZIFF M. EFFECTS OF THE RHEUMATOID FACTOR AND ANTIGEN-ANTIBODY COMPLEXES ON THE VESSELS OF THE RAT MESENTERY. J Immunol. 1964 Dec;93:985–992. [PubMed] [Google Scholar]
- Boesken W. H., Kopf K., Schollmeyer P. Differentiation of proteinuric diseases by discelectrophoretic molecular weight analysis of urinary proteins. Clin Nephrol. 1973 Sep-Oct;1(5):311–318. [PubMed] [Google Scholar]
- CHEN C. C., GROSSBERG A. L., PRESSMAN D. Effect of cyanate on several anti-hapten antibodies: evidence for the presence of an amino group in the site of anti-p-azobenzenearsonate antibody. Biochemistry. 1962 Nov;1:1025–1030. doi: 10.1021/bi00912a012. [DOI] [PubMed] [Google Scholar]
- DAVIS J. S., 4th, BOLLET A. J. PROTECTION OF A COMPLEMENT-SENSITIVE ENZYME SYSTEM BY RHEUMATOID FACTOR. J Immunol. 1964 Jan;92:139–144. [PubMed] [Google Scholar]
- DEBRO J. R., KORNER A. Solubility of albumin in alcohol after precipitation by trichloroacetic acid: a simplified procedure for separation of albumin. Nature. 1956 Nov 10;178(4541):1067–1067. doi: 10.1038/1781067a0. [DOI] [PubMed] [Google Scholar]
- DeHoratius R. J., Williams R. C., Jr Rheumatoid factor accentuation of pulmonary lesions associated with experimental diffuse proliferative lung disease. Arthritis Rheum. 1972 May-Jun;15(3):293–301. doi: 10.1002/art.1780150311. [DOI] [PubMed] [Google Scholar]
- EPSTEIN W. V., ENGLEMAN E. P. The relation of the rheumatoid factor content of serum to clinical neurovascular manifestations of rheumatoid arthritis. Arthritis Rheum. 1959 Jun;2(3):250–258. doi: 10.1002/1529-0131(195906)2:3<250::aid-art1780020306>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Fritz H., Husberg B. Ig-allotype-anti-Ig-allotype reaction in rabbit skin. Clin Exp Immunol. 1969 Jun;4(6):685–690. [PMC free article] [PubMed] [Google Scholar]
- Gordon D. A., Stein J. L., Broder I. The extra-articular features of rheumatoid arthritis. A systematic analysis of 127 cases. Am J Med. 1973 Apr;54(4):445–452. doi: 10.1016/0002-9343(73)90040-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McCormick J. N., Day J., Morris C. J., Hill A. G. The potentiating effect of rheumatoid arthritis serum in the immediate phase of nephrotoxic nephritis. Clin Exp Immunol. 1969 Jan;4(1):17–28. [PMC free article] [PubMed] [Google Scholar]
- Meltzer M., Franklin E. C. Cryoglobulinemia--a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966 Jun;40(6):828–836. doi: 10.1016/0002-9343(66)90199-9. [DOI] [PubMed] [Google Scholar]
- Mongan E. S., Cass R. M., Jacox R. F., Vaughen J. H. A study of the relation of seronegative and seropositive rheumatoid arthritis to each other and to necrotizing vasculitis. Am J Med. 1969 Jul;47(1):23–35. doi: 10.1016/0002-9343(69)90238-1. [DOI] [PubMed] [Google Scholar]
- PERNIS B., BALLABIO C. B., CHIAPPINO G. PRESENZA DEL FATTORE REUMATOIDE NELLE LESIONI VASCOLARI IN CASI DI ARTRITE REUMATOIDE AGGRAVATA (MALIGNA). STUDIO CON ANTICORPI FLUORESCENTI. Reumatismo. 1963 May-Jun;15:187–199. [PubMed] [Google Scholar]
- Riethmüller G., Meltzer M., Franklin E., Miescher P. A. Serum complement levels in patients with mixed (IgM-IgG) cryoglobulinaemia. Clin Exp Immunol. 1966 Jul;1(3):337–339. [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Tesar J. T., Schmid F. R. Conversion of soluble immune complexes into complement-fixing aggregates by IgM-rheumatoid factor. J Immunol. 1970 Nov;105(5):1206–1214. [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- WIEDERMANN G., MIESCHER P. A., FRANKLIN E. C. Effect of mercaptoethanol on complement binding ability of human 7 S gammaglobulin. Proc Soc Exp Biol Med. 1963 Jul;113:609–613. doi: 10.3181/00379727-113-28440. [DOI] [PubMed] [Google Scholar]
- Weber K., Ueki H., Wolff H. H., Braun-Falco O. Reversed passive arthus reaction using horseradish peroxidase as antigen. II. Light and electron microscopical observations. Arch Dermatol Forsch. 1974 Jul 1;250(1):15–32. doi: 10.1007/BF00558893. [DOI] [PubMed] [Google Scholar]
- Weisman M., Zvaifler N. Cryoimmunoglobulinemia in rheumatoid arthritis. Significance in serum of patients with rheumatoid vasculitis. J Clin Invest. 1975 Sep;56(3):725–739. doi: 10.1172/JCI108144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]


