Abstract
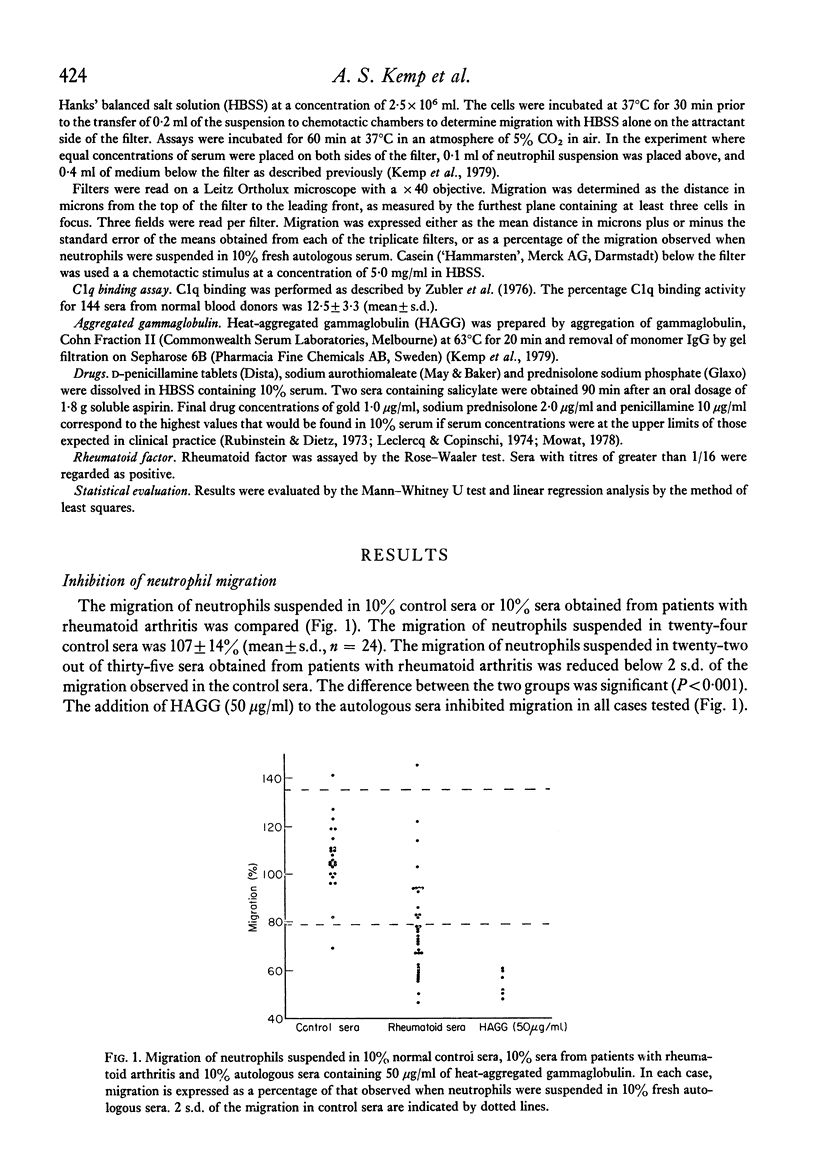
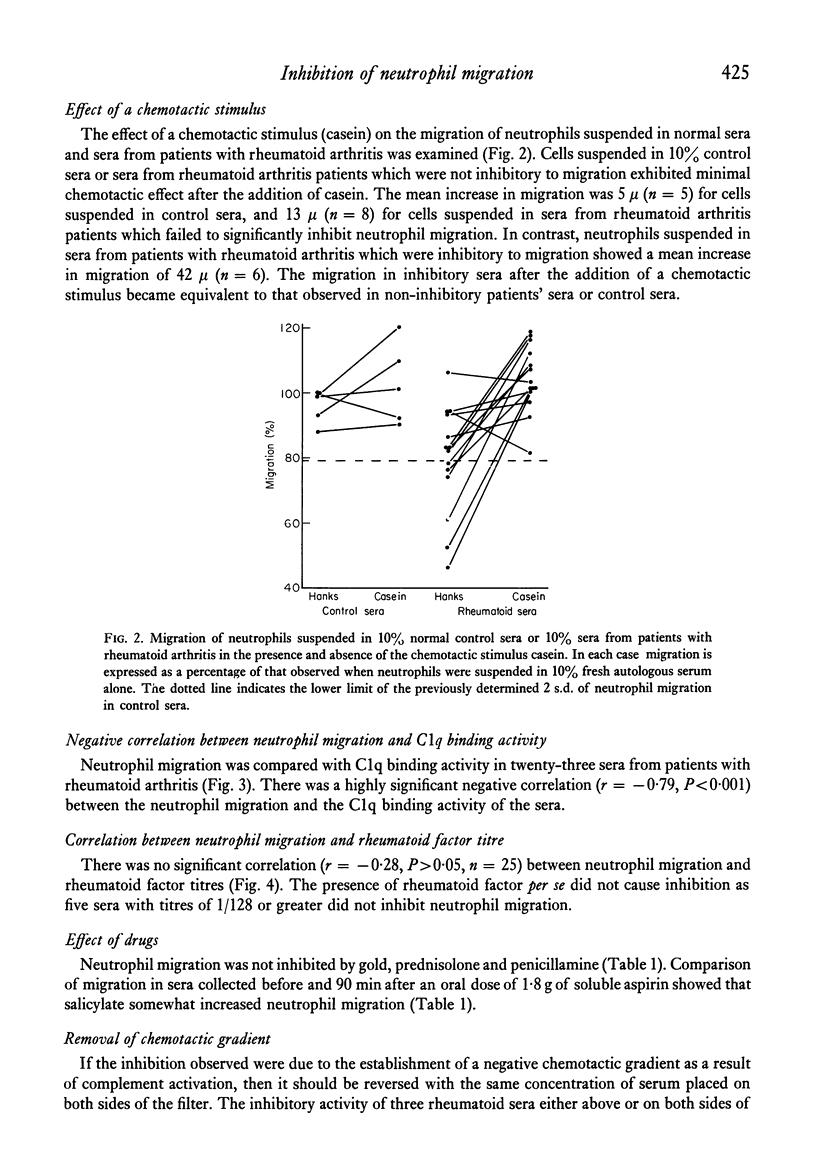
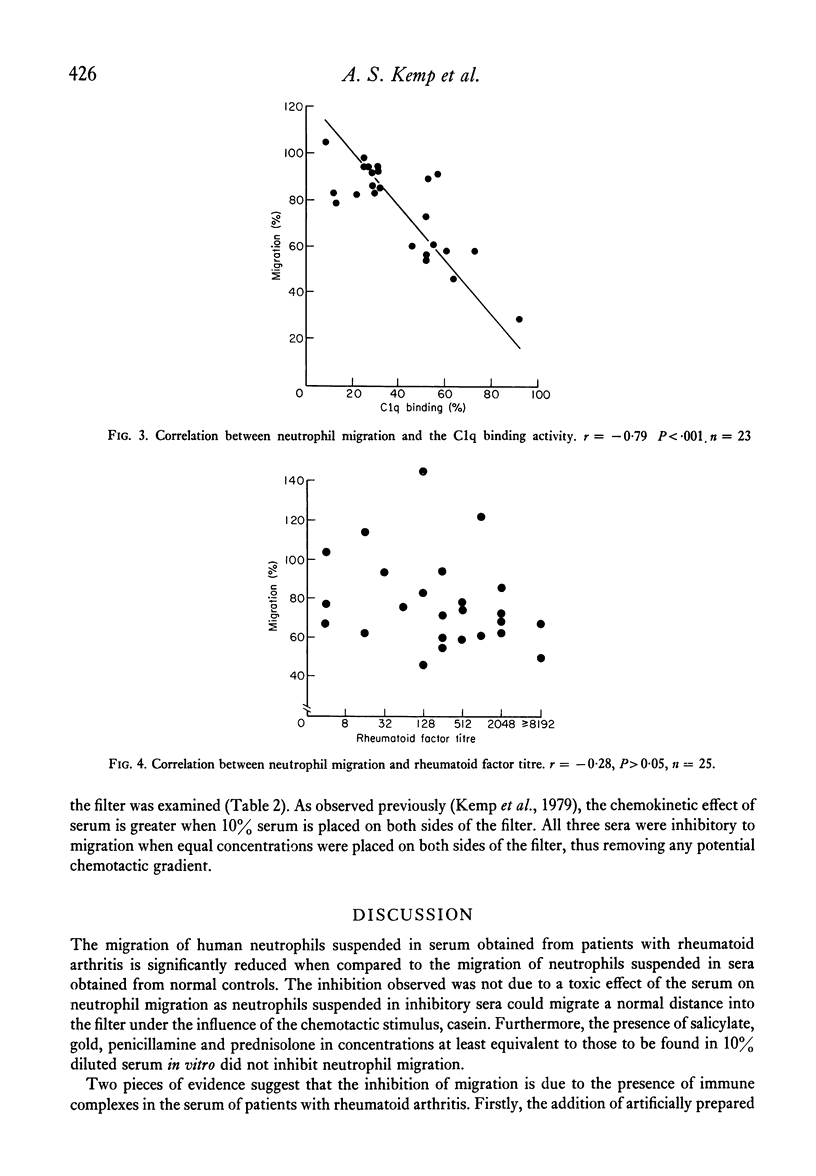
Sera from patients with rheumatoid arthritis inhibited the migration of human neutrophils in 63% (twenty-two out of thirty-five) of the cases tested. The inhibition was not due to a toxic effect of the serum as it was reversed by a chemotactic stimulus. There was a strong correlation between the degree of inhibition of neutrophil migration and the amount of immune complexes present in the sera as determined by the C1q binding activity. It is suggested that the inhibition of neutrophil migration is due to the presence of circulating immune complexes, and that the capacity of immune complexes to inhibit neutrophil migration in vitro may also contribute to the accumulation of neutrophils at sites of immune complex formation in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeuwkes H., Bijlsma A. Reduced chemotaxis of polymorphonuclear leukocytes in sera from patients with rheumatoid arthritis. Antonie Van Leeuwenhoek. 1974;40(2):233–239. doi: 10.1007/BF00394381. [DOI] [PubMed] [Google Scholar]
- Cats A., Lafeber G. J., Klein F. Immunoglobulin phagocytosis by granulocytes from sera and synovial fluids in various rheumatoid and nonrheumatoid diseases. Ann Rheum Dis. 1975 Apr;34(2):146–155. doi: 10.1136/ard.34.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad K. Presence of aggregated gamma-G-globulin in certain rheumatoid synovial effusions. Clin Exp Immunol. 1967 Jul;2(4):511–529. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Hess M. W., Cottier H. Physiology of chemotaxis and random motility. Semin Hematol. 1975 Jan;12(1):47–57. [PubMed] [Google Scholar]
- Kemp A., Roberts-Thomson P., Brown S. Inhibition of human neutrophil migration by aggregated gammaglobulin. Clin Exp Immunol. 1979 May;36(2):334–341. [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Copinschi G. Patterns of plasma levels of prednisolone after oral administration in man. J Pharmacokinet Biopharm. 1974 Apr;2(2):175–187. doi: 10.1007/BF01061507. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Howard A., Gotch F. M., Quie P. G. Effector activating determinants on IgG. I. The distribution and factors influencing the display of complement, neutrophil and cytotoxic B-cell determinants on human IgG sub-classes. Immunology. 1973 Sep;25(3):459–469. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with rheumatoid arthritis. J Clin Invest. 1971 Dec;50(12):2541–2549. doi: 10.1172/JCI106754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. G. Neutrophil chemotaxis in rheumatoid arthritis. Effect of D-penicillamine, gold salts, and levamisole. Ann Rheum Dis. 1978 Feb;37(1):1–8. doi: 10.1136/ard.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein H. M., Dietz A. A. Serum gold. II. Levels in rheumatoid arthritis. Ann Rheum Dis. 1973 Mar;32(2):128–132. doi: 10.1136/ard.32.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. A., Schumacher R., Myers A. R. Phagocytic function of polymorphonuclear leukocytes in rheumatic diseases. J Clin Invest. 1973 Jul;52(7):1632–1635. doi: 10.1172/JCI107342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T., Abraham G., Baum J. The roles of IgG, IgM rheumatoid factor, and their complexes in the induction of polymorphonuclear leukocyte chemotactic factor from complement. J Clin Invest. 1974 Jun;53(6):1503–1511. doi: 10.1172/JCI107700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Nydegger U., Perrin L. H., Fehr K., McCormick J., Lambert P. H., Miescher P. A. Circulating and intra-articular immune complexes in patients with rheumatoid arthritis. Correlation of 125I-Clq binding activity with clinical and biological features of the disease. J Clin Invest. 1976 May;57(5):1308–1319. doi: 10.1172/JCI108399. [DOI] [PMC free article] [PubMed] [Google Scholar]


