Abstract
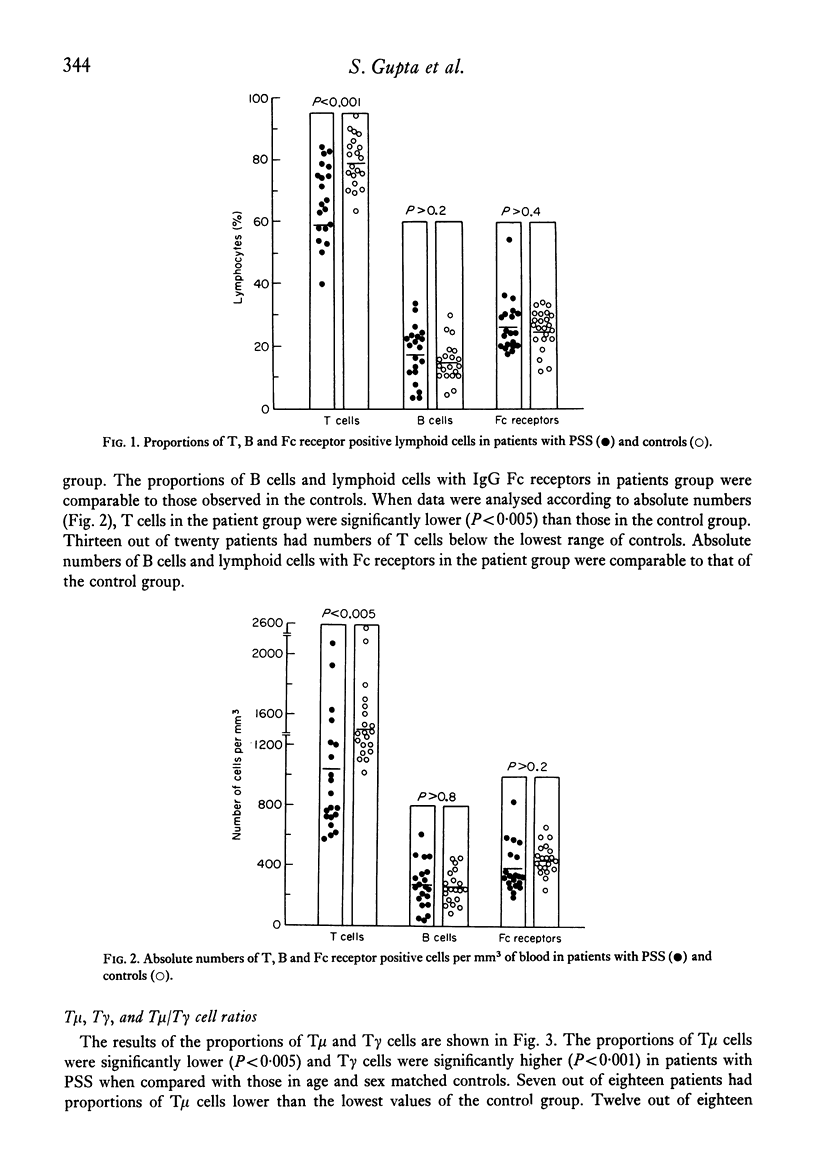
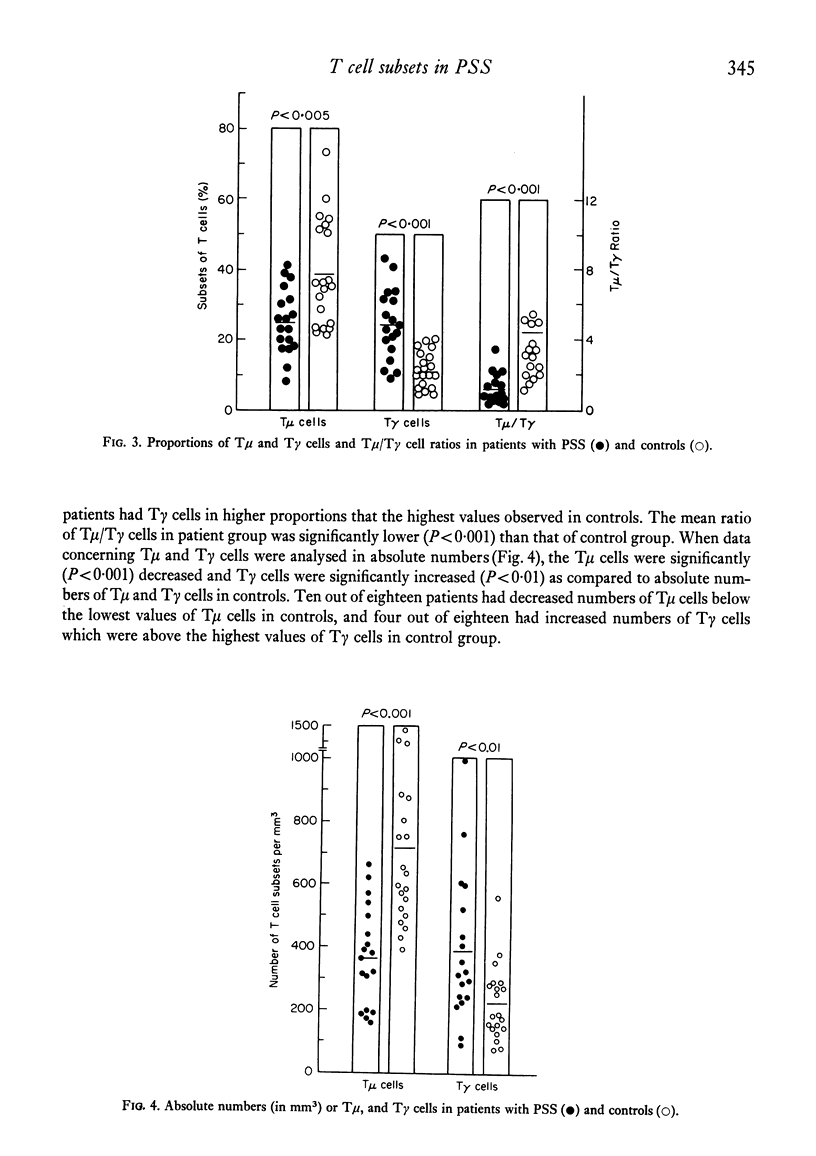
Peripheral blood lymphocytes from twenty patients with progressive systemic sclerosis (PSS) were analysed for the numbers and proportions of B lymphocytes possessing surface immunoglobulin, cells with Fc receptors, T cella and T cells with receptors for IgM (T mu) or IgG (T gamma). In patients with PSS, B cells and lymphocytes with Fc receptors were comparable in both numbers and proportions to those of the control group. Circulating T lymphocytes were significantly fewer in the patient group. T mu cells were decreased and T gamma cells increased, resulting in lower T mu/T gamma ratios as compared to controls. This study demonstrates a profound imbalance between T mu and T gamma cells (containing a population of helper or suppressor cells, respectively). These results are discussed in relation to immunodeficiencies observed in patients with PSS.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Currie S., Saunders M., Knowles M. Immunological aspects of systemic sclerosis in vitro activity of lymphocytes from patients with the disorder. Br J Dermatol. 1971 May;84(5):400–409. doi: 10.1111/j.1365-2133.1971.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Davis P., Jayson M. I. Serological changes in progressive systemic sclerosis. Rheumatol Rehabil. 1976 Feb;15(1):45–50. doi: 10.1093/rheumatology/15.1.45. [DOI] [PubMed] [Google Scholar]
- Gupta S., Fernandes G., Nair M., Good R. A. Spontaneous and antibody-dependent cell-mediated cytotoxicity by human T cell subpopulations. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5137–5141. doi: 10.1073/pnas.75.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. I. Studies in immunodeficient patients. Clin Exp Immunol. 1977 Nov;30(2):222–228. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. II. Effect of thymopoietin, corticosteroids, and irradiation. Cell Immunol. 1977 Nov;34(1):10–18. doi: 10.1016/0008-8749(77)90224-6. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. III. Distribution and quantitation in peripheral blood, cord blood, tonsils, bone marrow, thymus, lymph nodes, and spleen. Cell Immunol. 1978 Mar 15;36(2):263–270. doi: 10.1016/0008-8749(78)90270-8. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. V. T lymphocytes with receptors for immunoglobulin M or G in patients with primary immunodeficiency disorders. Clin Immunol Immunopathol. 1978 Nov;11(3):292–302. doi: 10.1016/0090-1229(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. X. Alterations in T, B, third population cells, and T cells with receptors for immunoglobulin M (Tmu) or G (Tgamma) in aging humans. J Immunol. 1979 Apr;122(4):1214–1219. [PubMed] [Google Scholar]
- Gupta S., Safai B., Good R. A. Subpopulations of human T lymphocytes. IV. Quantitation and distribution in patients with mycosis fungoides and Sézary syndrome. Cell Immunol. 1978 Aug;39(1):18–26. doi: 10.1016/0008-8749(78)90078-3. [DOI] [PubMed] [Google Scholar]
- Husson J. M., Druet P., Contet A., Fiessinger J. N., Camilleri J. P. Systemic sclerosis and cryoglobulinemia. Clin Immunol Immunopathol. 1976 Jul;6(1):77–82. doi: 10.1016/0090-1229(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Lockshin M. D., Eisenhauer A. C., Kohn R., Weksler M., Block S., Mushlin S. B. Cell-mediated immunity in rheumatic diseases. II. Mitogen responses in RA, SLE, and other illnesses: correlation with T- and B-lymphocyte populations. Arthritis Rheum. 1975 May-Jun;18(3):245–250. doi: 10.1002/art.1780180308. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem N. B., Morse J. H. Lymphocyte response to mitogens in progressive systemic sclerosis. Arthritis Rheum. 1976 Sep-Oct;19(5):875–882. doi: 10.1002/art.1780190507. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Postlethwaite A. E., Kang A. H. Evidence for cell-mediated immunity to collagen in progressive systemic sclerosis. J Lab Clin Med. 1976 Oct;88(4):601–607. [PubMed] [Google Scholar]
- Trompeter R. S., Layward L., Hayward A. R. Primary and secondary abnormalities of T cell subpopulations. Clin Exp Immunol. 1978 Dec;34(3):388–392. [PMC free article] [PubMed] [Google Scholar]
- Winkelstein A., Rodnan G. P., Heilman J. D. Cellular immunity in progressive systemic sclerosis (scleroderma). Ann Rheum Dis. 1972 Mar;31(2):126–128. doi: 10.1136/ard.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus D. G., Clancy R. L. Circulating T and B lymphocytes in progressive systemic sclerosis. J Rheumatol. 1975 Sep;2(3):336–339. [PubMed] [Google Scholar]


