Abstract
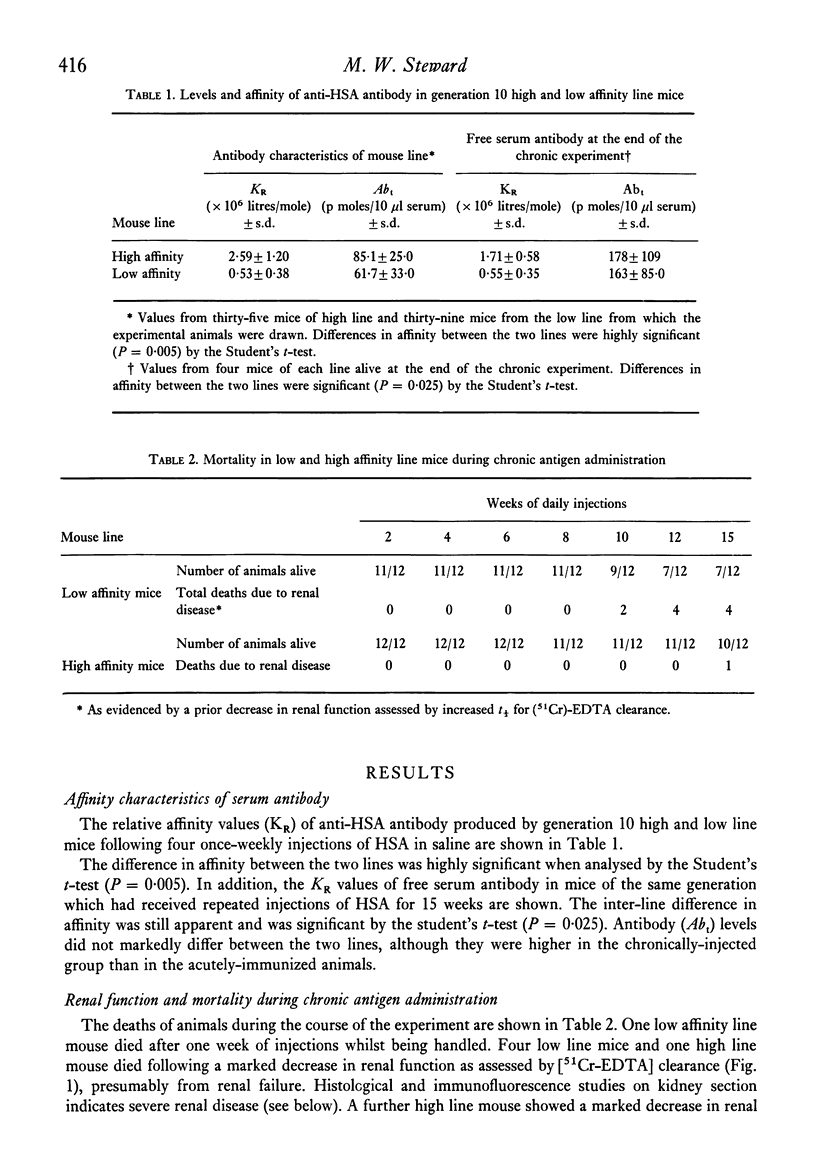
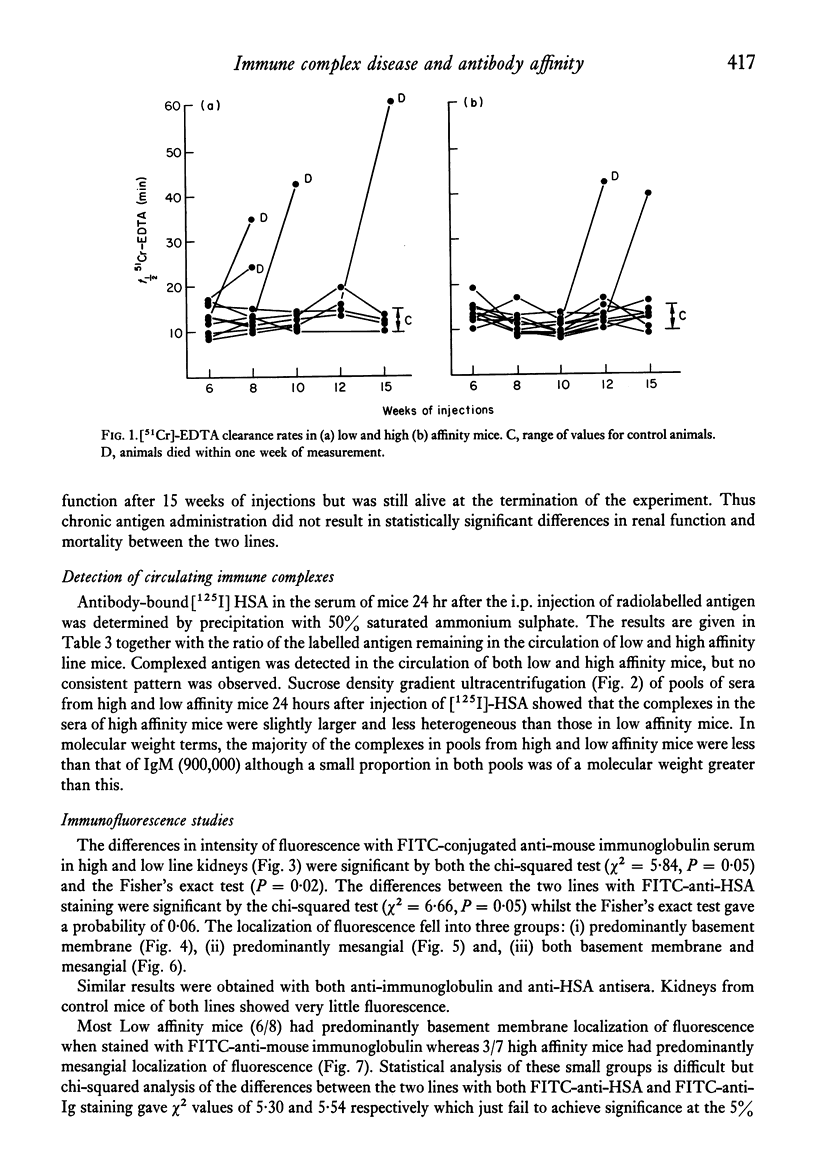
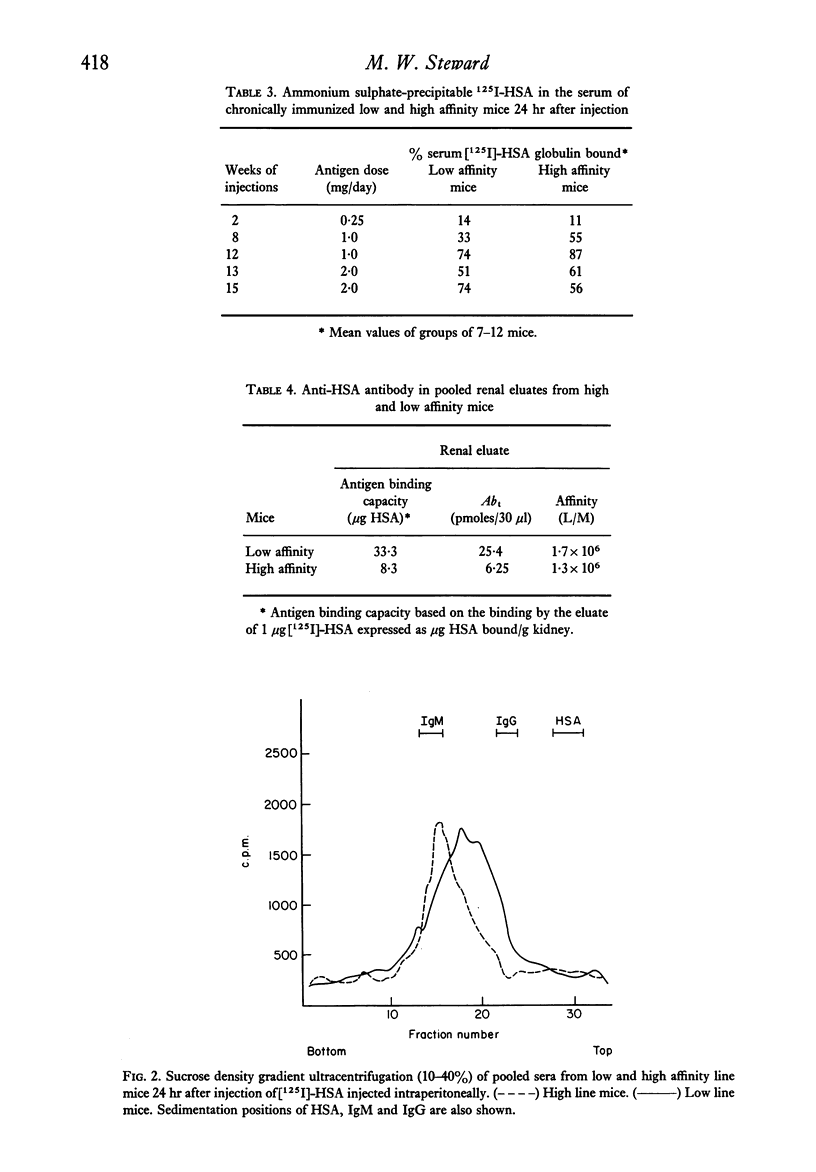
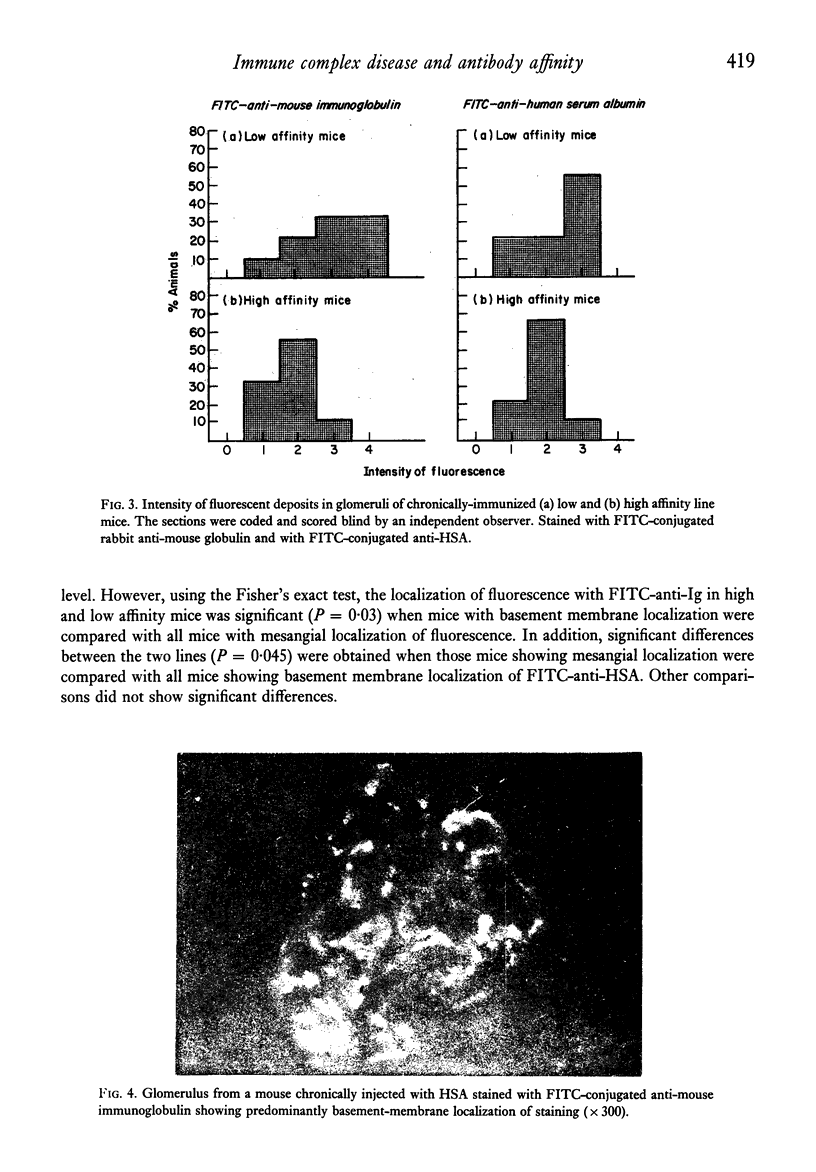
Two lines of mice selectively bred for producing high and low affinity antibody to protein antigens were repeatedly injected with human serum albumin and the severity and pattern of immune complex disease induced in this way was studied in the two lines. In low affinity mice, there was a greater intensity of deposits in the glomeruli shown by immunofluorescence, and more antibody was eluted from kidney homogenates compared to high affinity line mice. In the low affinity mice, complexes were mainly on the basement membrane whereas in high affinity mice, the localization of immune complexes was predominantly mesangial. However, no significant difference in glomerular filtration rates between the two lines was obtained. The immunopathological significance of antibody affinity is discussed in the light of these results.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers J. H., Steward M. W., Soothill J. F. Differences in immune elimination in inbred mice. The role of low affinity antibody. Clin Exp Immunol. 1972 Sep;12(1):121–132. [PMC free article] [PubMed] [Google Scholar]
- Appel G. B., Silva F. G., Pirani C. L., Meltzer J. I., Estes D. Renal involvement in systemic lupud erythematosus (SLE): a study of 56 patients emphasizing histologic classification. Medicine (Baltimore) 1978 Sep;57(5):371–410. doi: 10.1097/00005792-197809000-00001. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Mannik M. Studies on antigen-antibody complexes. II. Quantification of tissue uptake of soluble complexes in normal and complement-depleted rabbits. J Immunol. 1971 Jul;107(1):63–75. [PubMed] [Google Scholar]
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- Baldwin D. S., Gluck M. C., Lowenstein J., Gallo G. R. Lupus nephritis. Clinical course as related to morphologic forms and their transitions. Am J Med. 1977 Jan;62(1):12–30. doi: 10.1016/0002-9343(77)90345-x. [DOI] [PubMed] [Google Scholar]
- Bartolotti S. R. Quantitative elution studies in experimental immune complex and nephrotoxic nephritis. Clin Exp Immunol. 1977 Aug;29(2):334–341. [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Hawkins D. Studies on circulating immune complexes. 3. Factors governing the ability of circulating complexes to localize in blood vessels. J Exp Med. 1968 Jan 1;127(1):137–154. doi: 10.1084/jem.127.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. doi: 10.1016/s0065-2776(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. Mechanisms involved in the deposition of immune complexes in tissues. J Exp Med. 1971 Sep 1;134(3 Pt 2):75s–89s. [PubMed] [Google Scholar]
- DIXON F. J., FELDMAN J. D., VAZQUEZ J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med. 1961 May 1;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze S., West N. J., Steward M. W. The use of a double isotope method in the determination of antibody affinity. J Immunol Methods. 1973 Dec;3(4):357–364. doi: 10.1016/0022-1759(73)90037-9. [DOI] [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974 May;112(5):1939–1948. [PubMed] [Google Scholar]
- Katz F. E., Steward M. W. Studies on the genetic control of antibody affinity: the independent control of antibody levels and affinity in Biozzi mice. J Immunol. 1976 Aug;117(2):477–479. [PubMed] [Google Scholar]
- Katz F. E., Steward M. W. The genetic control of antibody affinity in mice. Immunology. 1975 Sep;29(3):543–548. [PMC free article] [PubMed] [Google Scholar]
- Kim Y. T., Siskind G. W. Studies on the control of antibody synthesis. XII. Genetic influences on antibody affinity. Immunology. 1978 Apr;34(4):669–678. [PMC free article] [PubMed] [Google Scholar]
- Knight J. G., Adams D. D., Purves H. D. The genetic contribution of the NZB mouse to the renal disease of the NZB x NZW hybrid. Clin Exp Immunol. 1977 May;28(2):352–358. [PMC free article] [PubMed] [Google Scholar]
- Koyama A., Niwa Y., Shigematsu H., Taniguchi M., Tada T. Studies on passive serum sickness. II. Factors determining the localization of antigen-antibody complexes in the murine renal glomerulus. Lab Invest. 1978 Mar;38(3):253–262. [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik M., Arend M. P., Hall A. P., Gilliland B. C. Studies on antigen-antibody complexes. I. Elimination of soluble complexes from rabbit circulation. J Exp Med. 1971 Apr 1;133(4):713–739. doi: 10.1084/jem.133.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passwell J. H., Steward M. W., Soothill J. F. Inter-mouse strain differences in macrophage function and its relationship to antibody responses. Clin Exp Immunol. 1974 May;17(1):159–167. [PMC free article] [PubMed] [Google Scholar]
- Petty R. E., Steward M. W., Soothill J. F. The heterogeneity of antibody affinity in inbred mice and its possible immunopathologic significance. Clin Exp Immunol. 1972 Oct;12(2):231–241. [PMC free article] [PubMed] [Google Scholar]
- Soothill J. F., Steward M. W. The immunopathological significance of the heterogeneity of antibody affinity. Clin Exp Immunol. 1971 Aug;9(2):193–199. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Hay F. C. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin Exp Immunol. 1976 Nov;26(2):363–370. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. Evidence for the genetic control of antibody affinity from breeding studies with inbred mouse strains producing high and low affinity antibody. Immunology. 1976 Jun;30(6):789–797. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Reinhardt M. C., Staines N. A. The genetic control of antibody affinity. Evidence from breeding studies with mice selectively bred for either high or low affinity antibody production. Immunology. 1979 Jul;37(3):697–703. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Dixon F. J. Experimental glomerulonephritis: immunological events and pathogenetic mechanisms. Adv Immunol. 1967;6:1–90. doi: 10.1016/s0065-2776(08)60521-0. [DOI] [PubMed] [Google Scholar]
- WEIGLE W. O. Elimination of antigen-antibody complexes from sera of rabbits. J Immunol. 1958 Sep;81(3):204–213. [PubMed] [Google Scholar]
- WOOD C., WHITE R. G. Experimental glomerulo-nephritis produced in mice by subcutaneous injections of heat-killed Proteus mirabilis. Br J Exp Pathol. 1956 Feb;37(1):49–60. [PMC free article] [PubMed] [Google Scholar]



