Abstract
Cellular immune responsiveness, as measured by lymphocyte transformation in one-way mixed leucocyte cultures (MLC) and in phytohaemagglutinin (PHA) stimulated cultures was evaluated in forty patients with systemic lupus erythematosus (SLE) and in seventy-four normal controls. The effect produced by sera from these subjects on in vitro lymphocyte reactivity was tested on autologous cells and on homologous responding cells from a constant panel of ten healthy volunteers.
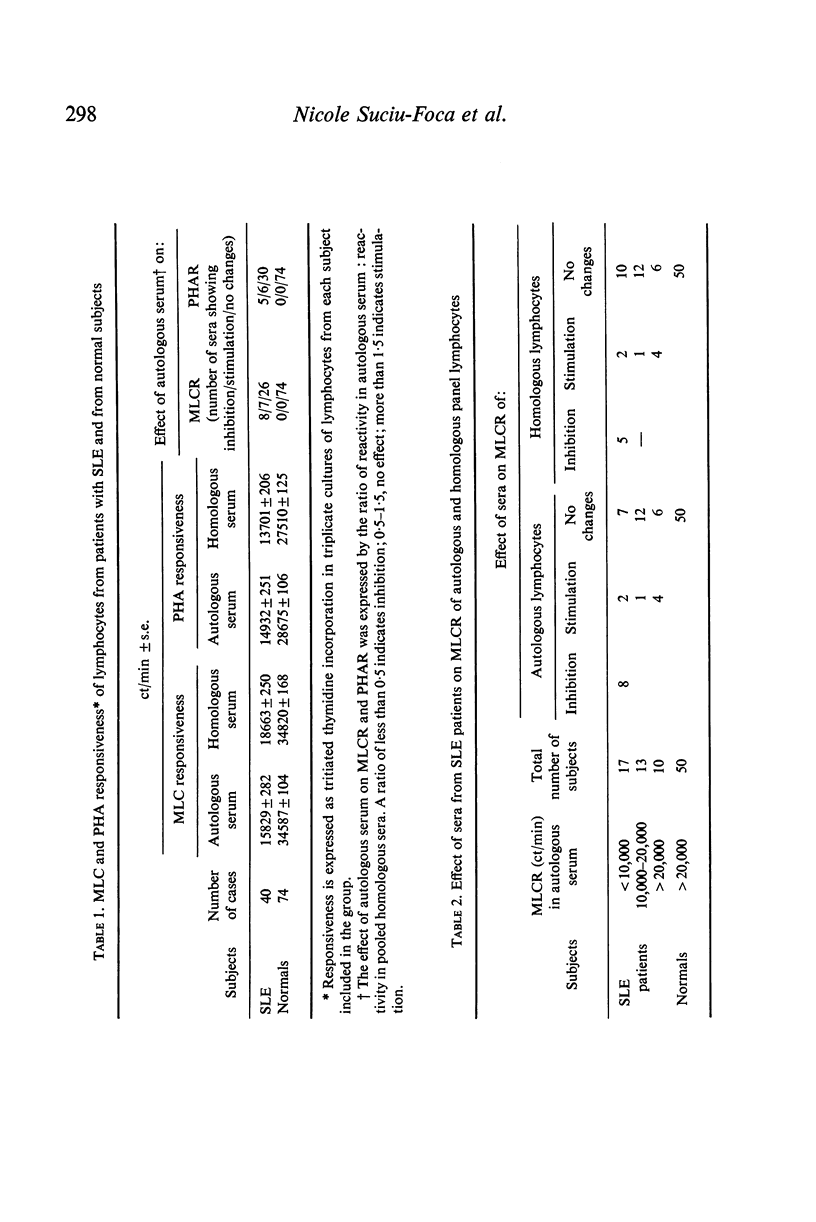
The reactivity of lymphocytes from SLE patients to PHA and to a battery of allogeneic cells was significantly lower than that of normal controls.
Sera from some SLE patients inhibited the MLC reactions, while in other cases a distinct stimulatory effect was found.
It is suggested that virus-induced modifications of normal histocompatibility antigens cause the appearance of blocking antibody that might bind to the surface of T lymphocytes, impairing their function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Astorga G. P., Williams R. C., Jr Altered reactivity in mixed lymphocyte culture of lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1969 Dec;12(6):547–554. doi: 10.1002/art.1780120602. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Schiff R. I., Amos D. B. Blocking of autologous and homologous leukocyte responses by human alloimmune plasmas: a possible in vitro correlate of enhancement. J Immunol. 1972 Jan;108(1):34–44. [PubMed] [Google Scholar]
- Ceppellini R., Bonnard G. D., Coppo F., Miggiano V. C., Pospisil M., Curtoni E. S., Pellegrino M. Transplantation antigens: introductory symposium. Mixed leukocyte cultures and HL-A antigens. I. Reactivity of young fetuses, newborns and mothers at delivery. Transplant Proc. 1971 Mar;3(1):58–63. [PubMed] [Google Scholar]
- Feldman J. D. Immunological enhancement: a study of blocking antibodies. Adv Immunol. 1972;15:167–214. doi: 10.1016/s0065-2776(08)60685-9. [DOI] [PubMed] [Google Scholar]
- Gatti R. A., Yunis E. J., Good R. A. Characterization of a serum inhibitor of MLC reactions. Clin Exp Immunol. 1973 Mar;13(3):427–437. [PMC free article] [PubMed] [Google Scholar]
- Hattler B. G., Jr, Karesh C., Miller J. Inhibition of the mixed lymphocyte culture response by antibody following successful human renal transplantation. Tissue Antigens. 1971;1(6):270–275. doi: 10.1111/j.1399-0039.1971.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Hedberg H., Källén B., Löw B., Nilsson O. Impaired mixed leucocyte reaction in some different diseases, notably multiple sclerosis and various arthritides. Clin Exp Immunol. 1971 Aug;9(2):201–207. [PMC free article] [PubMed] [Google Scholar]
- Klippel J. H., Grimley P. M., Decker J. L. Lymphocyte inclusions in newborns of mothers with systemic lupus erythematosus. N Engl J Med. 1974 Jan 10;290(2):96–97. doi: 10.1056/NEJM197401102900208. [DOI] [PubMed] [Google Scholar]
- Lueker R. D., Williams R. C., Jr Decreased reactivity of lymphocytes in mixed-leukocyte culture from patients with rheumatic fever. Circulation. 1972 Oct;46(4):655–660. doi: 10.1161/01.cir.46.4.655. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengar D. P., Opelz G., Terasaki P. I. Outcome of kidney transplants and suppression of mixed leukocyte culture by plasma. Transplant Proc. 1973 Mar;5(1):641–647. [PubMed] [Google Scholar]
- Suciu-Foca N., Buda J., McManus J., Thiem T., Reemtsma K. Impaired responsiveness of lymphocytes and serum-inhibitory factors in patients with cancer. Cancer Res. 1973 Oct;33(10):2373–2377. [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lies R. B., Messner R. P. Inhibition of mixed leukocyte culture responses by serum and gamma-globulin fractions from certain patients with connective tissue disorders. Arthritis Rheum. 1973 Sep-Oct;16(5):597–605. doi: 10.1002/art.1780160504. [DOI] [PubMed] [Google Scholar]


