Abstract
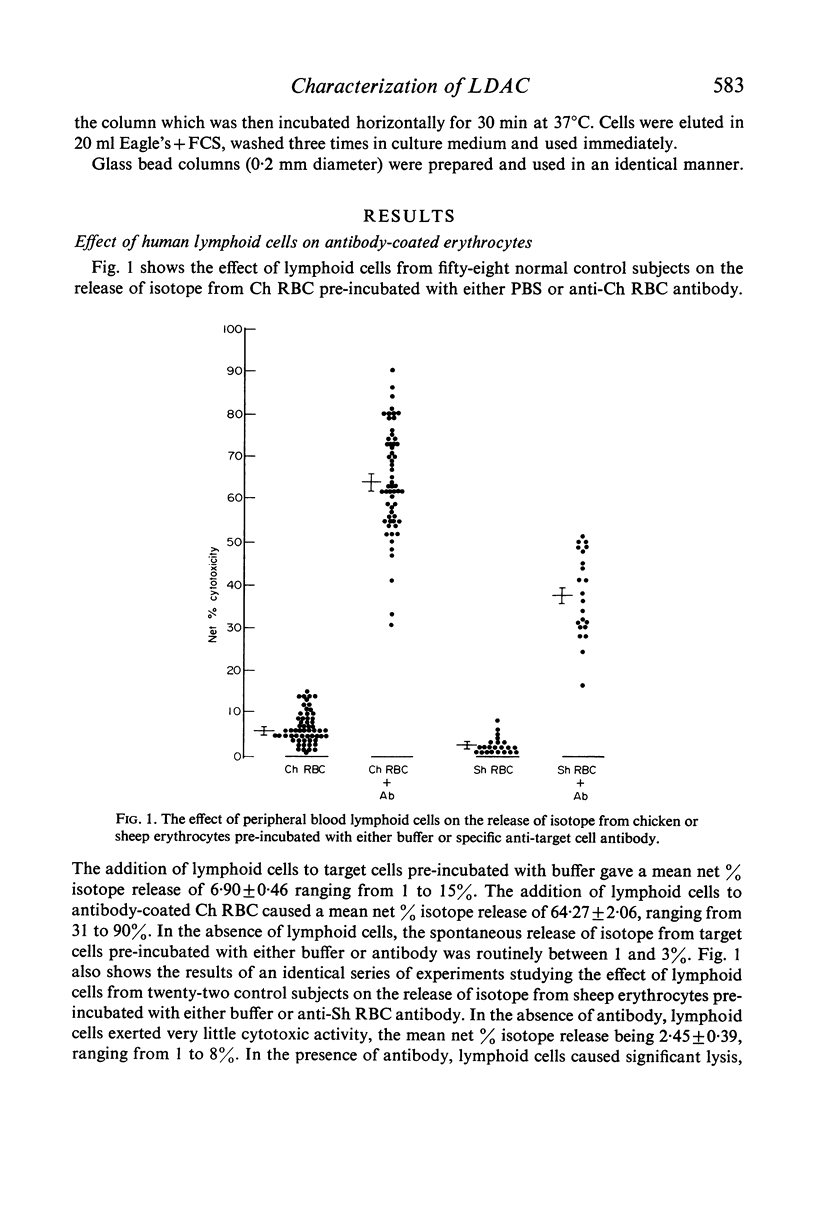
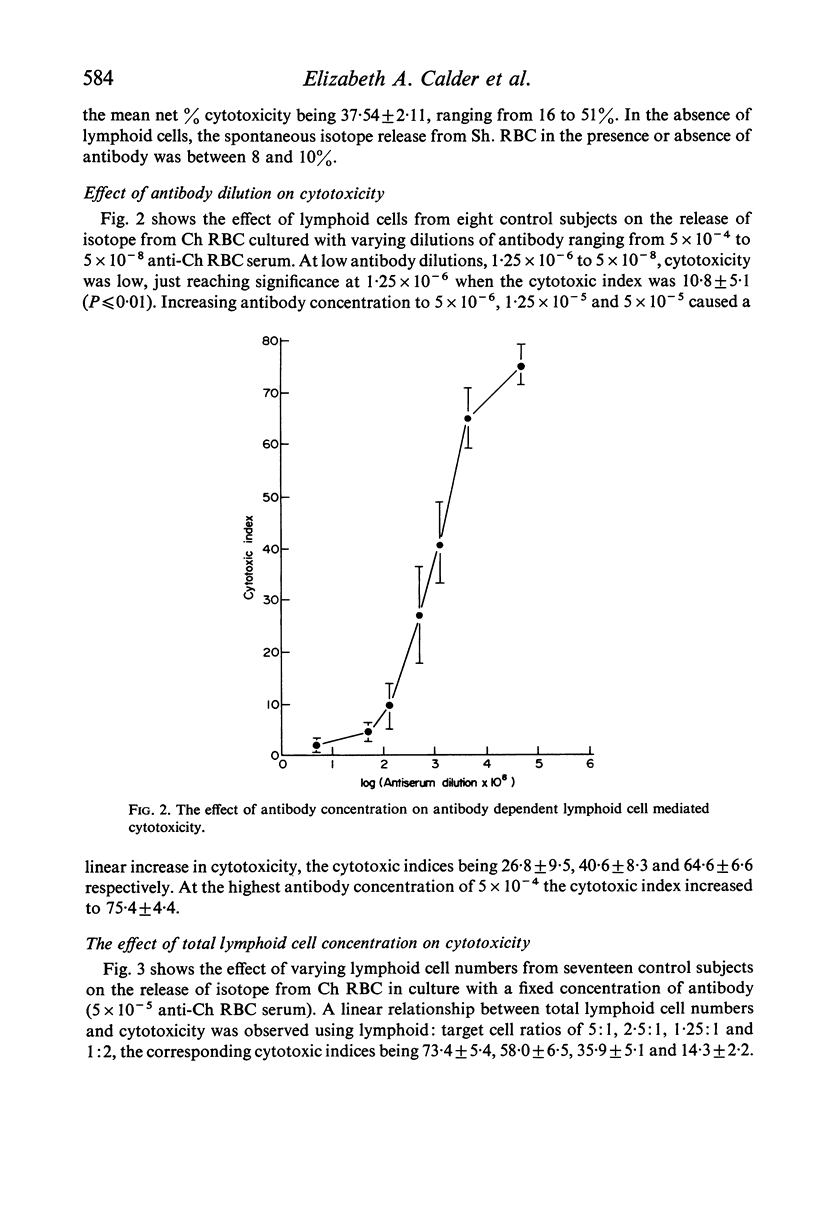
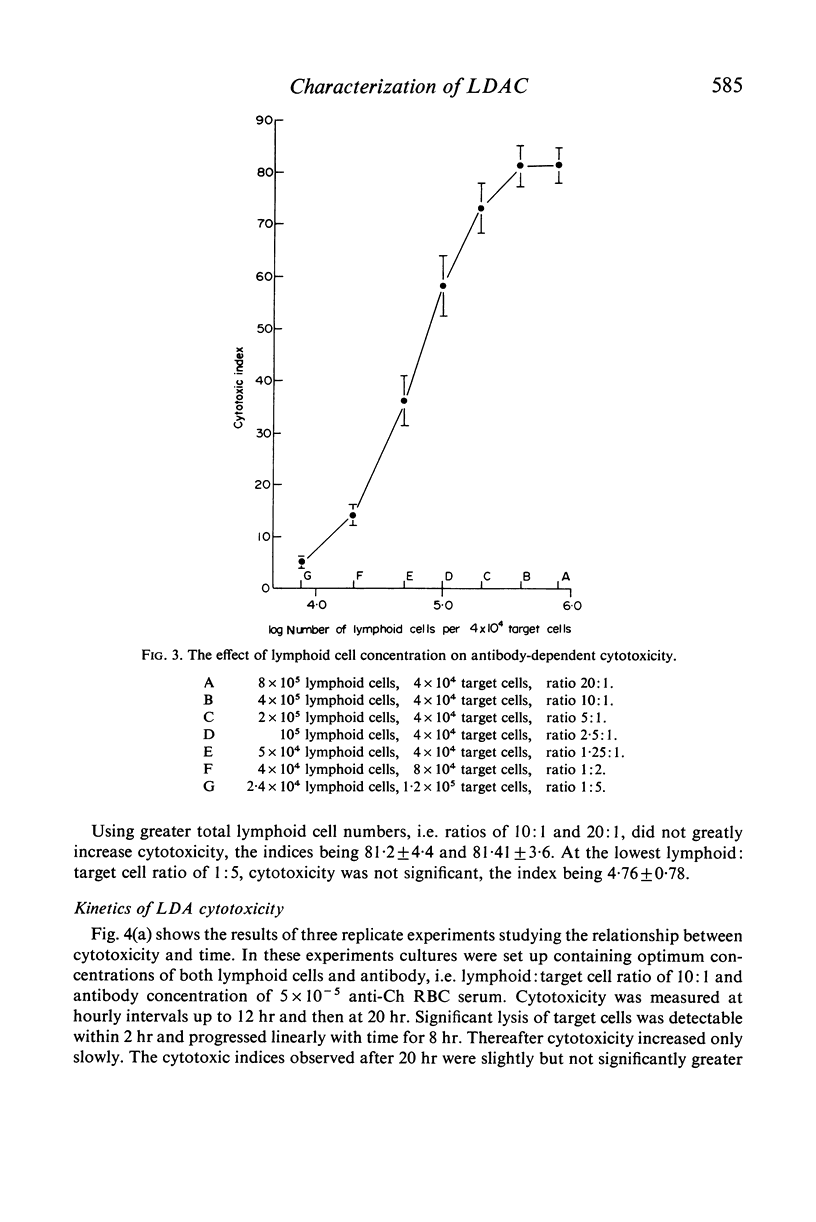
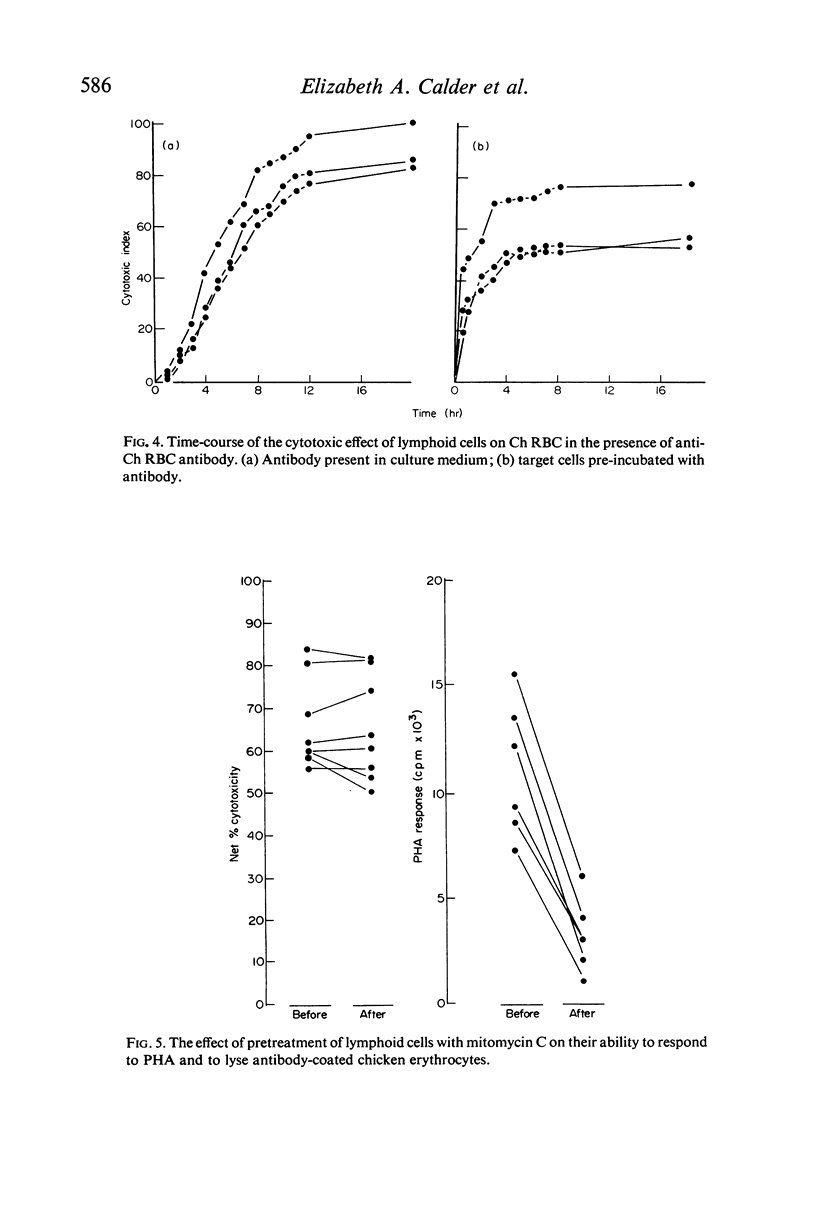
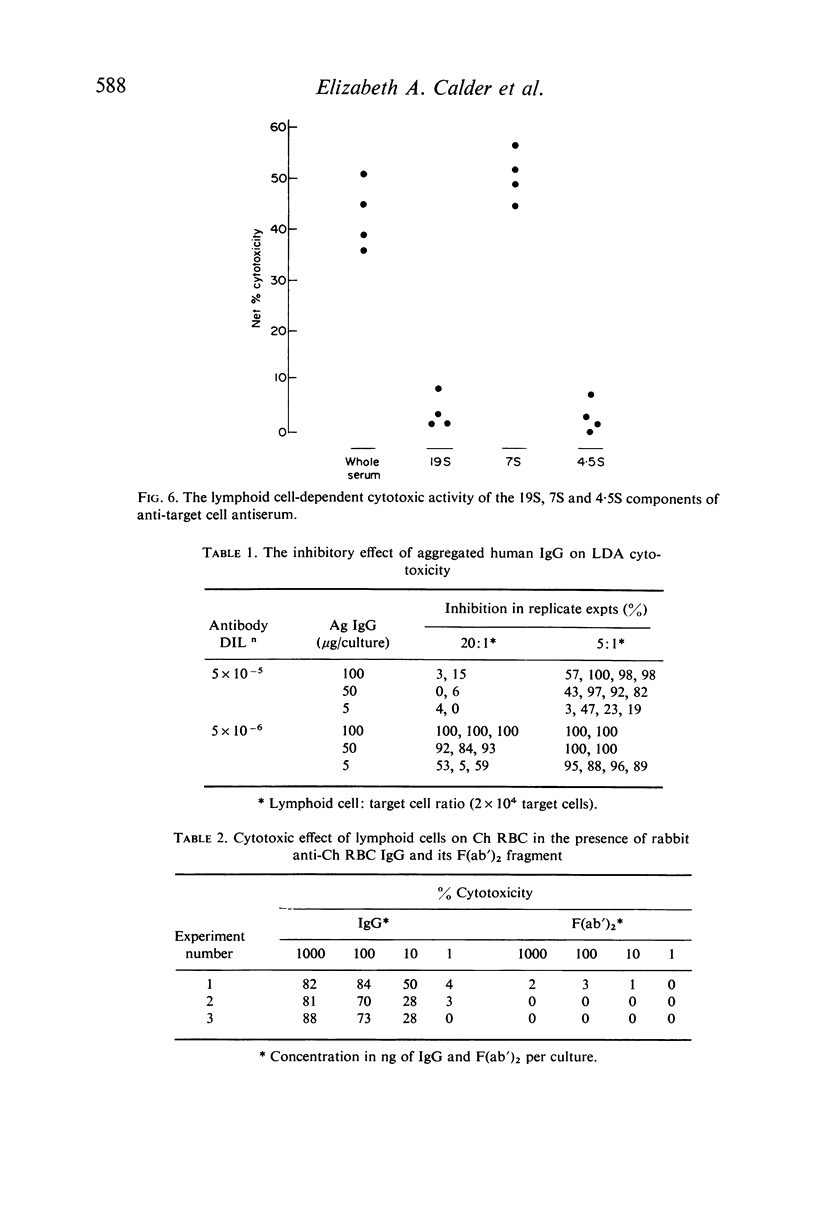
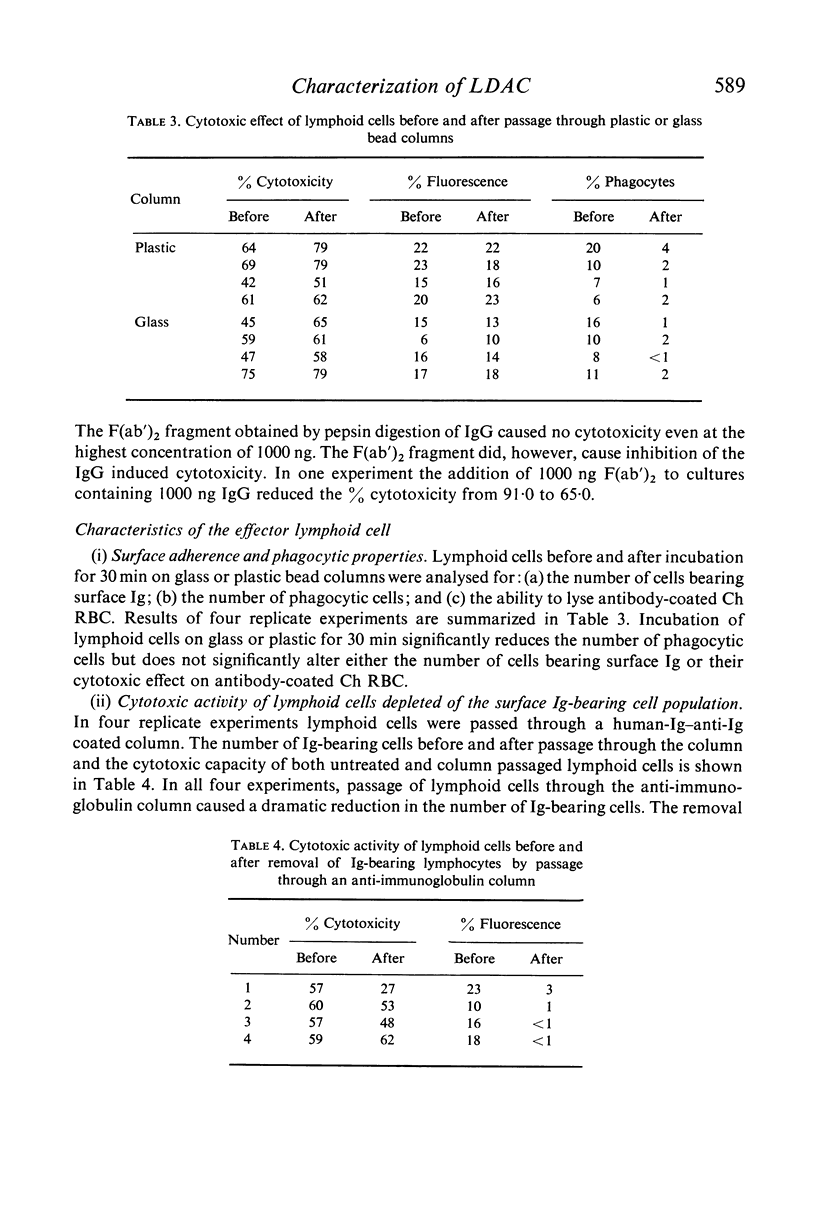
Cytotoxicity was studied in a model system using chicken erythrocytes (Ch RBC) labelled with 51Cr as target cells and human peripheral blood lymphoid cells as effector cells. In vitro human lymphoid cells are highly efficient in destroying target cells coated with anti-target cell antibody, the mean net percentage cytotoxicity of lymphoid cells from fifty-eight control subjects being 64·27±2·06 (SEM). In the absence of antibody the mean net percentage cytotoxicity was 6·90±0·46. As little as 10 ng rabbit anti-Ch RBC IgG was required to cause significant target cell lysis. Studies on the nature of the lymphoid cell-dependent cytotoxic antibody showed that it is localized in the 7S IgG region of whole serum and that an intact Fc region is required; the F(ab')2 fragment obtained by pepsin digestion of IgG was inactive although able to inhibit the cytotoxic activity of the whole undigested IgG. Investigation of the kinetics of LDAC showed that when antibody was added to the final culture medium target cell lysis progressed rapidly (detectable within 2 hr) and linearly with time up to 8 hr. Thereafter the rate of lysis decreased reaching a maximum after 12 hr culture. With cultures containing target cells which have been pre-incubated with antibody, lysis occurred even more rapidly, detectable within 30 min and reaching a maximum after only 3–4 hr culture. The maximum cytotoxicity in this system was, however, lower than that obtained when antibody was added directly to the culture medium. Cytotoxicity could be inhibited by the addition of aggregated human IgG, as little as 5 μg causing 100% inhibition of target cell lysis. Study of the nature of the effector lymphoid cell showed, first, that viable cells were required, twice frozen/thawed lymphoid cell suspensions being inactive; secondly, that active protein synthesis by the effector cell was not an essential prerequisite, pretreatment of lymphoid cells with mitomycin C having no significan effect on their ability to lyse antibody-coated target cells but significantly reducing their ability to transform in response to the mitogen PHA; thirdly, that the effector cell is non-phagocytic, non-plastic or glass-adherent and does not bear surface immunoglobulin; and fourthly that significant cytotoxicity is detectable even with a total lymphoid cell to target cell ratio of 1:2.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bubeník J., Perlmann P., Hasek M. Induction of cytotoxicity of lymphocytes from tolerant donors by antibodies to target cell alloantigens. Transplantation. 1970 Oct;10(4):290–296. [PubMed] [Google Scholar]
- Calder B. A., Penhale W. J., Barnes E. W., Irvine W. J. Cytotoxic lymphocytes in Hashimoto thyroiditis. An in vitro assay system using 51 Cr-labelled chicken red blood cells coated with thyroglobulin. Clin Exp Immunol. 1973 May;14(1):19–23. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. In vitro cytotoxic activity of thymus cells sensitized to alloantigens. Nature. 1970 Jul 4;227(5253):72–73. doi: 10.1038/227072a0. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Golstein P., Wigzell H., Blomgren H., Svedmyr E. A. Cells mediating specific in vitro cytotoxicity. II. Probable autonomy of thymus-processed lymphocytes (T cells) for the killing of allogeneic target cells. J Exp Med. 1972 Apr 1;135(4):890–906. doi: 10.1084/jem.135.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. H., Hudson L., Shen L., Roitt I. M. Antibody-dependent cell-mediated cytotoxicity due to a "null" lymphoid cell. Nat New Biol. 1973 Mar 28;242(117):111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- Greenberg A. H., Shen L., Roitt I. M. Characterization of the antibody-dependent cytotoxic cell. A non-phagocytic monocyte? Clin Exp Immunol. 1973 Oct;15(2):251–259. [PMC free article] [PubMed] [Google Scholar]
- Hallberg T. Inhibition of cytotoxicity of nonimmune human lymphocytes for sensitized chicken erythrocytes by aggregated human IgG. Scand J Immunol. 1974;3(1):117–125. doi: 10.1111/j.1365-3083.1974.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Harding B., Pudifin D. J., Gotch F., MacLennan I. C. Cytotoxic lymphocytes from rats depleted of thymus processed cells. Nat New Biol. 1971 Jul 21;232(29):80–82. doi: 10.1038/newbio232080a0. [DOI] [PubMed] [Google Scholar]
- James K. The preparation and properties of anti-lymphocytic sera. Prog Surg. 1969;7:140–217. doi: 10.1159/000386301. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A., Perlmann P. Study of Fab and F(ab') 2 from rabbit IgG for capacity to induce lymphocyte-mediated target cell destruction in vitro. Int Arch Allergy Appl Immunol. 1972;43(1):80–88. doi: 10.1159/000230823. [DOI] [PubMed] [Google Scholar]
- Lundgren G., Collste L., Möller G. Cytotoxicity of human lymphocytes: antagonism between inducing processes. Nature. 1968 Oct 19;220(5164):289–291. doi: 10.1038/220289a0. [DOI] [PubMed] [Google Scholar]
- MOELLER E. CONTACT-INDUCED CYTOTOXICITY BY LYMPHOID CELLS CONTAINING FOREIGN ISOANTIGENS. Science. 1965 Feb 19;147(3660):873–879. doi: 10.1126/science.147.3660.873. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Connell G. E., Gotch F. M. Effector activating determinants on IgG. II. Differentiation of the combining sites for C1q from those for cytotoxic K cells and neutrophils by plasmin digestion of rabbits IgG. Immunology. 1974 Feb;26(2):303–310. [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Harding B. Failure of certain cytotoxic lymphocytes to respond mitotically to phytohaemagglutinin. Nature. 1970 Sep 19;227(5264):1246–1248. doi: 10.1038/2271246a0. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Harding B. The role of immunoglobulins in lymphocyte-mediated cell damage, in vitro. II. The mechanism of target cell damage by lymphoid cells from immunized rats. Immunology. 1970 Mar;18(3):405–412. [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Loewi G. Effect of specific antibody to target cells on their specific and non-specific interactions with lymphocytes. Nature. 1968 Sep 7;219(5158):1069–1070. doi: 10.1038/2191069a0. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Loewi G., Harding B. The role of immunoglobulins in lymphocyte-mediated cell damage, in vitro. I. Comparison of the effects of target cell specific antibody and normal serum factors on cellular damage by immune and non-immune lymphocytes. Immunology. 1970 Mar;18(3):397–404. [PMC free article] [PubMed] [Google Scholar]
- Penhale W. J., Farmer A., Maccuish A. C., Irvine W. J. A rapid micro-method for the phytohaemagglutinin-induced human lymphocyte transformation test. Clin Exp Immunol. 1974 Sep;18(1):155–167. [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Wigzell H. Lymphocyte mediated cytotoxicity in vitro. Induction and inhibition by humoral antibody and nature of effector cells. Transplant Rev. 1972;13:91–114. doi: 10.1111/j.1600-065x.1972.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Stjernswärd J., Jondal M., Vánky F., Wigzell H., Sealy R. Lymphopenia and change in distribution of human B and T lymphocytes in peripheral blood induced by irradiation for mammary carcinoma. Lancet. 1972 Jun 24;1(7765):1352–1356. doi: 10.1016/s0140-6736(72)91091-4. [DOI] [PubMed] [Google Scholar]
- Urbaniak S. J., Penhale W. J., Irvine W. J. Circulating lymphocyte subpopulations in Hashimoto thyroiditis. Clin Exp Immunol. 1973 Nov;15(3):345–354. [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Stobo J. D., Paul W. E., Green I. Antibody-dependent lymphoid cell-mediated cytotoxicity: no requirement for thymus-derived lymphocytes. Science. 1972 Jan 14;175(4018):194–196. doi: 10.1126/science.175.4018.194. [DOI] [PubMed] [Google Scholar]
- Wasserman J., Packalén T., Perlmann P., Perlmann H. Cytotoxic lymphoid cells and antibodies from guinea pigs immunized with tubercle bacilli. Int Arch Allergy Appl Immunol. 1969;36(1):115–116. doi: 10.1159/000230730. [DOI] [PubMed] [Google Scholar]
- Wisloff F., Froland S. S. Antibody-dependent lymphocyte-mediated cytotoxicity in man: no requirement for lymphocytes with membrane-bound immunoglobulin. Scand J Immunol. 1973;2(2):151–157. doi: 10.1111/j.1365-3083.1973.tb02026.x. [DOI] [PubMed] [Google Scholar]


