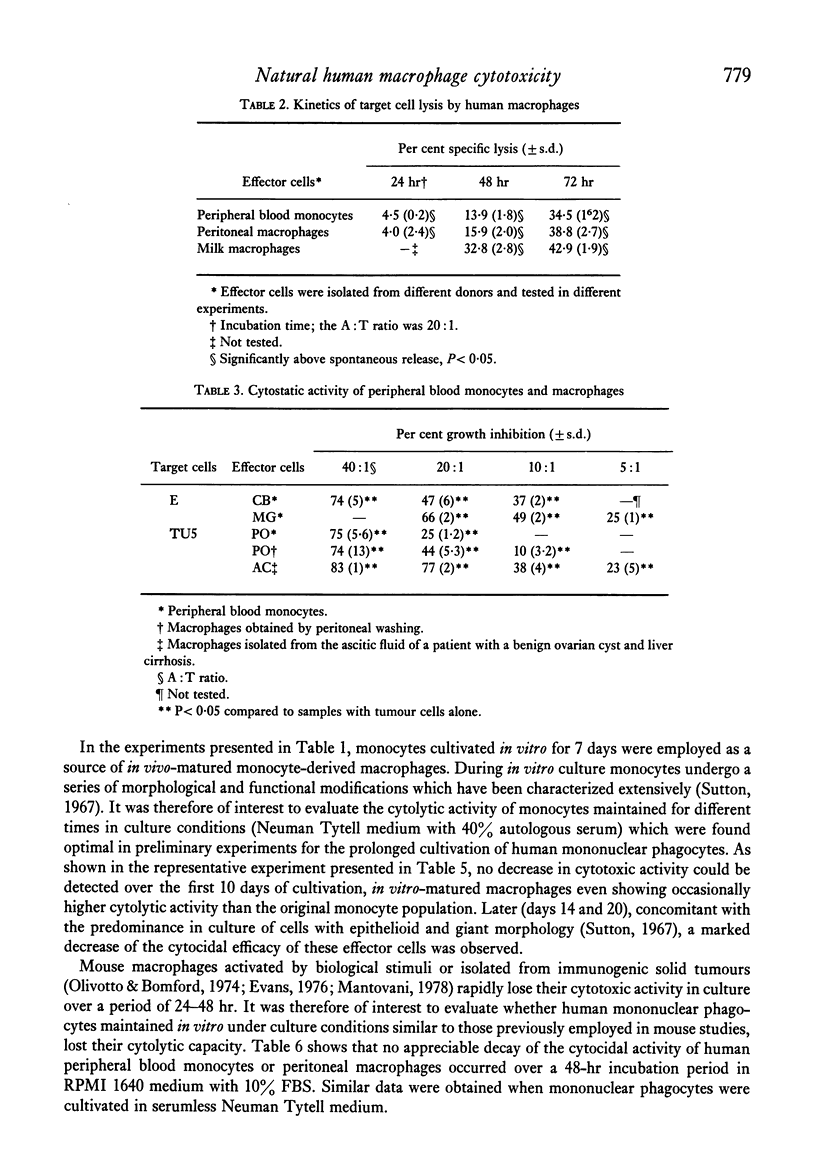
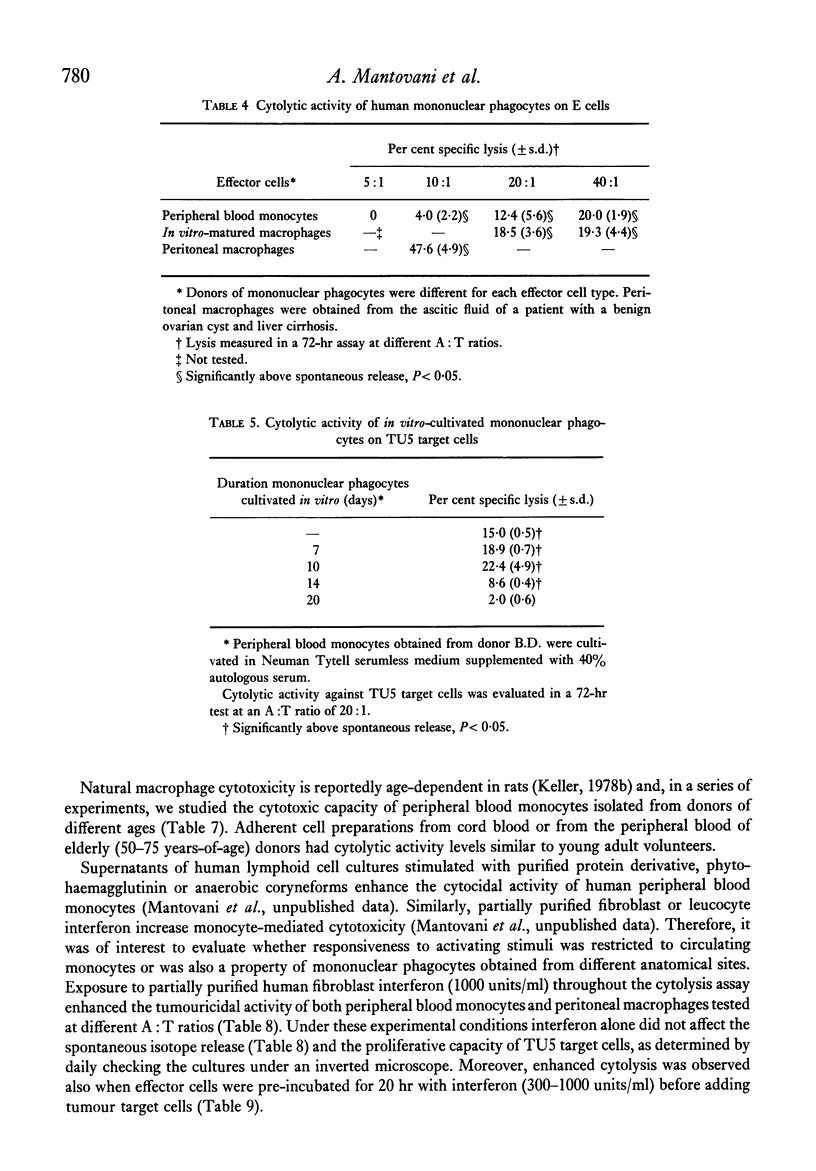
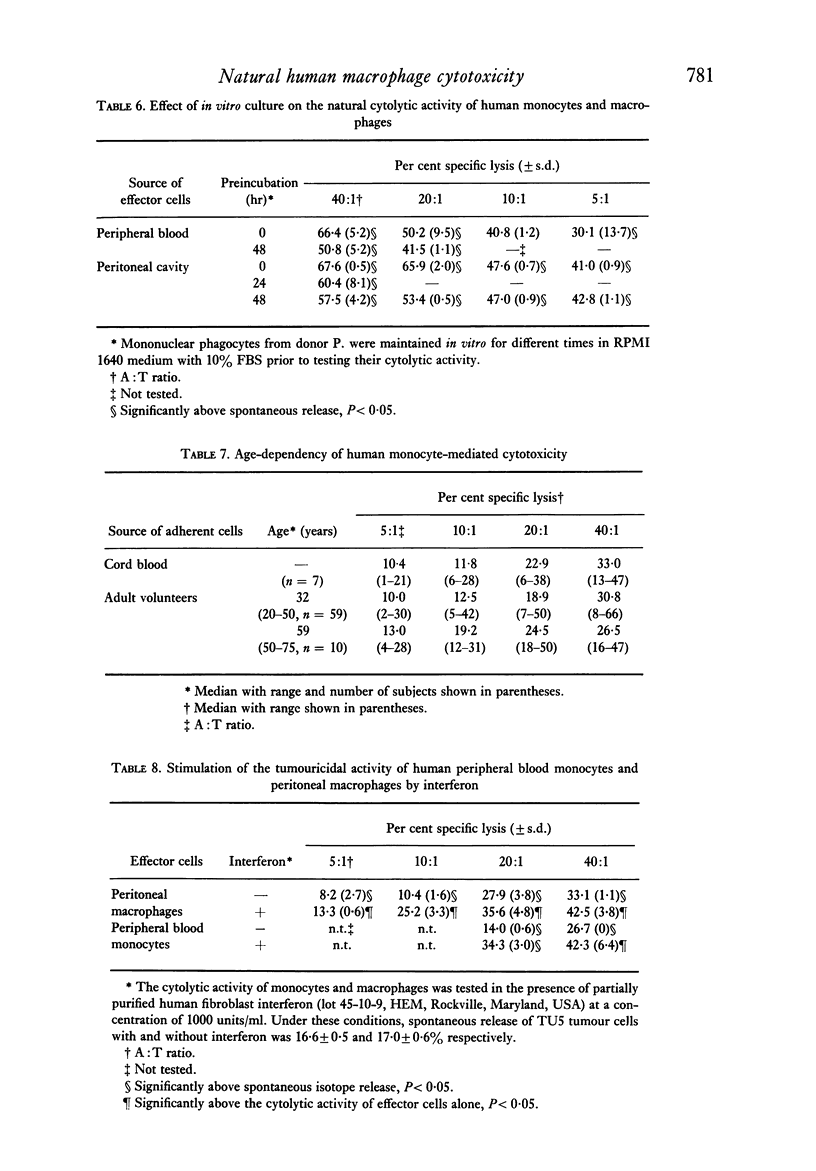
Abstract
Human mononuclear phagocytes were isolated from peripheral blood, peritoneal exudate and early lactation milk by adherence on microexudate-coated plastic and exposure to ethylene diamine tetracetic acid. Their cytolytic activity was measured as 3H-thymidine release from prelabelled target cells over 48-72 hr and cytostasis was evaluated in a spectrophotometric 72-hr assay. The murine SV40-transformed mKSA-TU5 line and the human E cell line, derived from an ovarian carcinoma, were employed as targets. Peripheral blood monocytes, in vitro-matured monocyte-derived macrophages, peritoneal macrophages and milk macrophages were all significantly cytolytic and cytostatic on these target cells at attacker to target cell ratios ranging from 5:1 to 40:1. When monocytes were cultivated in vitro, no loss of cytocidal capacity occurred over the first 10 days of culture, whereas later on, when epithelioid and giant cells predominate in the cultures, mononuclear phagocytes had little cytotoxic activity. Adherent cells obtained from cord blood or from the peripheral blood of old donors had natural cytotoxicity similar to monocytes obtained from young adult volunteers. Peripheral blood monocytes and peritoneal macrophages showed enhanced cytolytic activity after exposure to partially purified human fibroblast interferon. These experiments suggest that in the human mononuclear phagocyte series cytotoxicity on tumour cells is not restricted to circulating monocytes but is also expressed by macrophages obtained from diverse anatomical sites.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birbeck M. S., Carter R. L. Observations on the ultrastructure of two hamster lymphomas with particular reference to infiltrating macrophages. Int J Cancer. 1972 Mar 15;9(2):249–257. doi: 10.1002/ijc.2910090202. [DOI] [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Kight N., Temple A., Allison A. C. Spontaneous and induced cytotoxic properties of human adherent mononuclear cells: killing of non-sensitized and antibody-coated non-erythroid cells. Immunology. 1979 Feb;36(2):221–228. [PMC free article] [PubMed] [Google Scholar]
- Keller R., Keist R., Ivatt R. J. Functional and biochemical parameters of activation related to macrophage cytostatic effects on tumor cells. Int J Cancer. 1974 Nov 15;14(5):675–683. doi: 10.1002/ijc.2910140515. [DOI] [PubMed] [Google Scholar]
- Keller R. Macrophage-mediated natrual cytotoxicity against various target cells in vitro. I. Macrophages from diverse anatomical sites and different strains of rats and mice. Br J Cancer. 1978 May;37(5):732–741. doi: 10.1038/bjc.1978.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Macrophage-mediated natural cytotoxicity against various target cells in vitro. II. Macrophages from rats of different ages. Br J Cancer. 1978 May;37(5):742–746. doi: 10.1038/bjc.1978.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Caprioli V., Gritti P., Spreafico F. Human mature macrophages mediate antibody-dependent cellular cytotoxicity on tumour cells. Transplantation. 1977 Oct;24(4):291–293. doi: 10.1097/00007890-197710000-00010. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Effects on in vitro tumor growth of murine macrophages isolated from sarcoma lines differing in immunogenicity and metastasizing capacity. Int J Cancer. 1978 Dec;22(6):741–746. doi: 10.1002/ijc.2910220617. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Jerrells T. R., Dean J. H., Herberman R. B. Cytolytic and cytostatic activity on tumor cells of circulating human monocytes. Int J Cancer. 1979 Jan 15;23(1):18–27. doi: 10.1002/ijc.2910230105. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Tagliabue A., Dean J. H., Jerrells T. R., Herberman R. B. Cytolytic activity of circulating human monocytes on transformed and untransformed human fibroblasts. Int J Cancer. 1979 Jan 15;23(1):28–31. doi: 10.1002/ijc.2910230106. [DOI] [PubMed] [Google Scholar]
- Martin F., Martin M., Jeannin J. F., Lagneau A. Rat macrophage-mediated toxicity to cancer cells; effect of endotoxins and endotoxin inhibitors contained in culture media. Eur J Immunol. 1978 Aug;8(8):607–611. doi: 10.1002/eji.1830080813. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Miller G. A., Feldman J. D. Genetic role of rat macrophage cytotoxicity against tumor. Int J Cancer. 1976 Aug 15;18(2):168–175. doi: 10.1002/ijc.2910180206. [DOI] [PubMed] [Google Scholar]
- Olivotto M., Bomford R. In vitro inhibition of tumour cell growth and DNA synthesis by peritoneal and lung macrophages from mice injected with Corynebacterium parvum. Int J Cancer. 1974 Apr 15;13(4):478–488. doi: 10.1002/ijc.2910130406. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Kerbel R. S. An assessment of intratumor phagocytic and surface marker-bearing cells in a series of autochthonous and early passaged chemically induced murine sarcomas. J Natl Cancer Inst. 1976 Nov;57(5):1157–1167. doi: 10.1093/jnci/57.5.1157. [DOI] [PubMed] [Google Scholar]
- Russell S. W., McIntosh A. T. Macrophages isolated from regressing Moloney sarcomas are more cytotoxic than those recovered from progressing sarcomas. Nature. 1977 Jul 7;268(5615):69–71. doi: 10.1038/268069a0. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Papamatheakis J. D., Chirigos M. A. Interferon: an inducer of macrophage activation by polyanions. Science. 1977 Aug 12;197(4304):674–676. doi: 10.1126/science.877584. [DOI] [PubMed] [Google Scholar]
- Sutton J. S. Ultrastructural aspects of in vitro development of monocytes into macrophages, epithelioid cells, and multinucleated giant cells. Natl Cancer Inst Monogr. 1967 Sep;26:71–141. [PubMed] [Google Scholar]
- Tagliabue A., Mantovani A., Kilgallen M., Herberman R. B., McCoy J. L. Natural cytotoxicity of mouse monocytes and macrophages. J Immunol. 1979 Jun;122(6):2363–2370. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Tilly R. Some properties of cells cultured from early-lactation human milk. J Natl Cancer Inst. 1977 Jun;58(6):1563–1571. doi: 10.1093/jnci/58.6.1563. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gillespie G. Y. Studies on the role of macrophages in regulation of growth and metastasis of murine chemically induced fibrosarcomas. Int J Cancer. 1975 Dec 15;16(6):1022–1029. doi: 10.1002/ijc.2910160616. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Transplantation immunity to simian virus 40-transformed cells in tumor-bearing mice. II. Evidence for macrophage participation at the effector level of tumor cell rejection. J Natl Cancer Inst. 1973 Jan;50(1):149–157. doi: 10.1093/jnci/50.1.149. [DOI] [PubMed] [Google Scholar]


