Abstract
The possible pathogenetic role of lymphocytes sensitized to liver antigens was investigated in CBA mice in which sublethal hepatic necrosis had been induced by carbon tetrachloride (CCl4). Sensitized lymphocytes from CCl4-treated mice were administered to syngeneic recipients. The recipients developed sensitivity to liver antigens but showed no evidence of liver damage. The cell mediating the immune response both in the donor and the recipient was a T cell. This was demonstrated further by studies involving mice rendered T cell deficient. These mice did not develop sensitized lymphocytes when they were treated with CCl4 but the extent of liver damage was similar in both T cell-depleted and intact animals. These findings suggest that T cell sensitization to liver antigens occurs as a result of toxic liver damage and does not play a role in the pathogenesis of the hepatic necrosis.
Full text
PDF

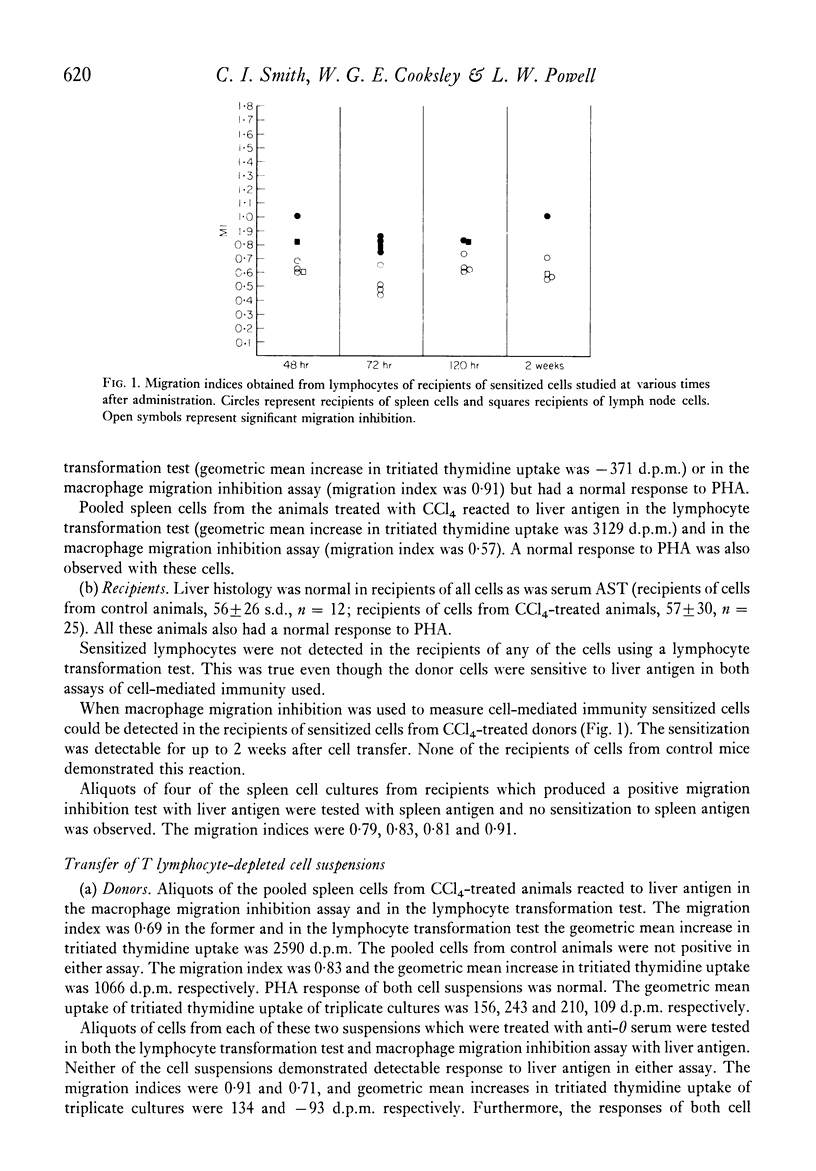
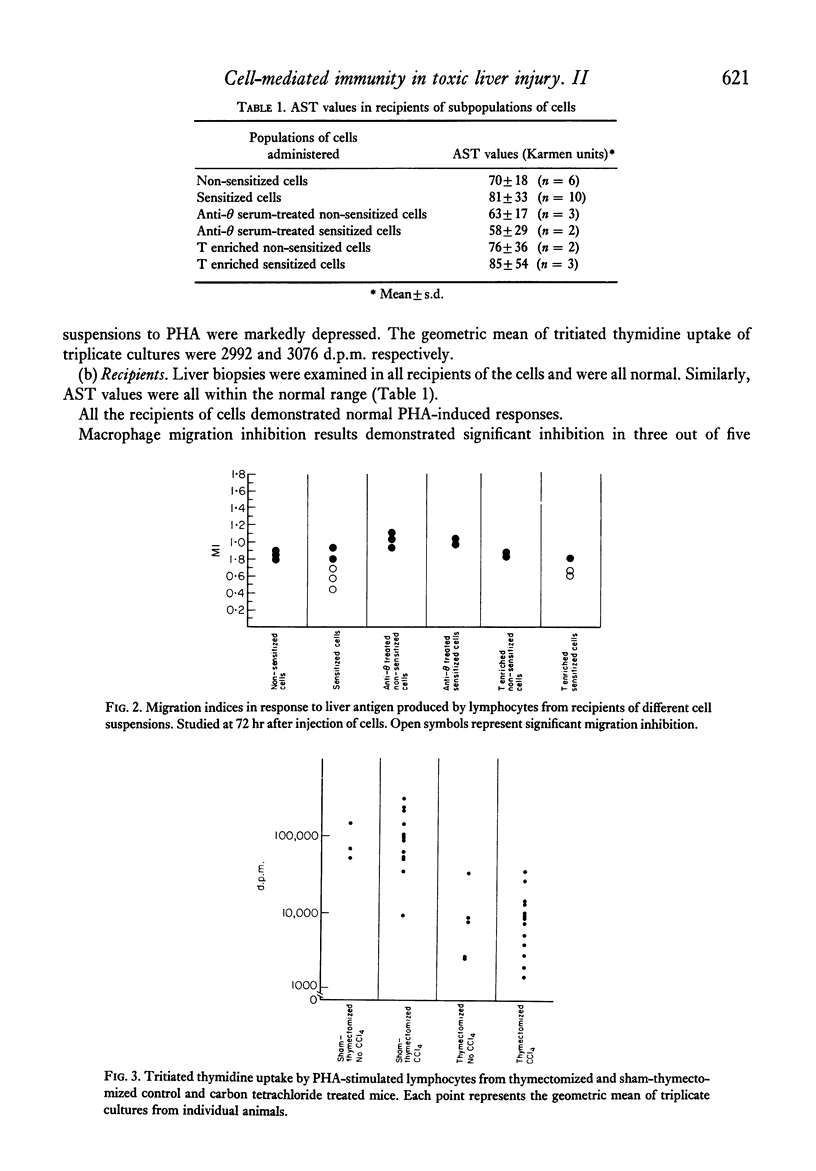

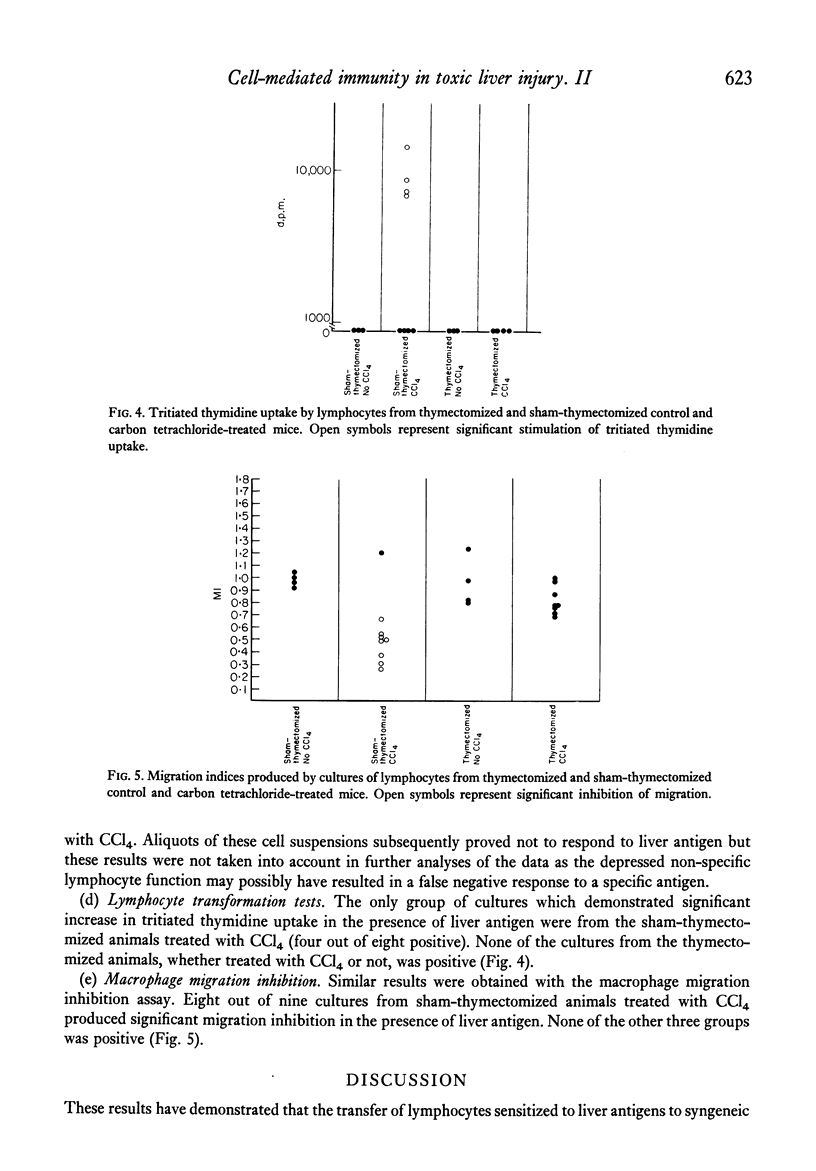


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane A. M., Moussouros A., Portmann B., McFarlane I. G., Thomson A. D., Eddleston, Williams R. Lymphocyte cytotoxicity for isolated hepatocytes in alcoholic liver disease. Gastroenterology. 1977 May;72(5 Pt 1):918–923. [PubMed] [Google Scholar]
- Cochrane A. M., Moussouros A., Thomsom A. D., Eddleston A. L., Wiiliams R. Antibody-dependent cell-mediated (K cell) cytotoxicity against isolated hepatocytes in chronic active hepatitis. Lancet. 1976 Feb 28;1(7957):441–444. doi: 10.1016/s0140-6736(76)91472-0. [DOI] [PubMed] [Google Scholar]
- Dickmeiss E., Soeberg B., Svejgaard A. Human cell-mediated cytotoxicity against modified target cells is restricted by HLA. Nature. 1977 Dec 8;270(5637):526–528. doi: 10.1038/270526a0. [DOI] [PubMed] [Google Scholar]
- Jensen D. M., McFarlane I. G., Portmann B. S., Eddleston A. L., Williams R. Detection of antibodies directed against a liver-specific membrane lipoprotein in patients with acute and chronic active hepatitis. N Engl J Med. 1978 Jul 6;299(1):1–7. doi: 10.1056/NEJM197807062990101. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- MILLER J. F. Studies on mouse leukaemia. The role of the thymus in leukaemogenesis by cell-free leukaemic filtrates. Br J Cancer. 1960 Mar;14:93–98. doi: 10.1038/bjc.1960.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaurin B. P. Controlled study of autoimmunization against liver in inbred rat strains. Gut. 1971 Jun;12(6):458–464. doi: 10.1136/gut.12.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane I. G., Wojcicka B. M., Zucker G. M., Eddleston A. L., Williams R. Purification and characterization of human liver-specific membrane lipoprotein (LSP). Clin Exp Immunol. 1977 Mar;27(3):381–390. [PMC free article] [PubMed] [Google Scholar]
- Miller J., Smith M. G., Mitchell C. G., Reed W. D., Eddleston A. L., Williams R. Cell-mediated immunity to a human liver-specific antigen in patients with active chronic hepatitis and primary biliary cirrhosis. Lancet. 1972 Aug 12;2(7772):296–297. doi: 10.1016/s0140-6736(72)92904-2. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffarth F., Warnatz H., Mayer K. Studies concerning the importance of mononuclear cells in the development of experimental hepatitis. J Immunol. 1967 Feb;98(2):396–401. [PubMed] [Google Scholar]
- Scheiffarth F., Warnatz H., Schmidt H. J. Immunological studies on the development of experimental hepatitis in thymectomized mice. Int Arch Allergy Appl Immunol. 1967;32(3):308–317. doi: 10.1159/000229941. [DOI] [PubMed] [Google Scholar]
- Smith C. I., Cooksley W. G., Powell L. W. Cell-mediated immunity to liver antigen in toxic liver injury. I. Occurrence and specificity. Clin Exp Immunol. 1980 Mar;39(3):607–617. [PMC free article] [PubMed] [Google Scholar]
- Smith C. I., Cooksley W. G., Powell L. W. Immunological abnormalities associated with liver disease: cause or effect? Aust N Z J Med. 1977 Dec;7(6):604–612. doi: 10.1111/j.1445-5994.1977.tb02316.x. [DOI] [PubMed] [Google Scholar]
- Tobias H., Safran A. P., Schaffner F. Lymphocyte stimulation and chronic liver disease. Lancet. 1967 Jan 28;1(7483):193–195. doi: 10.1016/s0140-6736(67)91830-2. [DOI] [PubMed] [Google Scholar]
- Warnatz H., Scheiffarth F., Liebelt E. Studien zur Ubertragbarkeit der experimentellen Hepatitis im Parabioseversuch. Z Immunitatsforsch Allerg Klin Immunol. 1968 Jul;136(1):60–67. [PubMed] [Google Scholar]
- Warnatz H., Scheiffarth F., Schmeissner R. Studies on the cytotoxic effect of in vivo and in vitro immunized lymphocytes on liver target cells. Clin Exp Immunol. 1975 Aug;21(2):250–258. [PMC free article] [PubMed] [Google Scholar]
- Warnatz H., Scheiffarth F., Wolf F., Schmidt H. J. Autoradiographic experiments concerning the importance of mononuclear cells in experimental hepatitis. J Immunol. 1967 Feb;98(2):402–405. [PubMed] [Google Scholar]
- Zatz M. M., Lance E. M. The distribution of chromium 51-labelled lymphoid cells in the mouse. A survey of anatomical compartments. Cell Immunol. 1970 May;1(1):3–17. doi: 10.1016/0008-8749(70)90057-2. [DOI] [PubMed] [Google Scholar]


