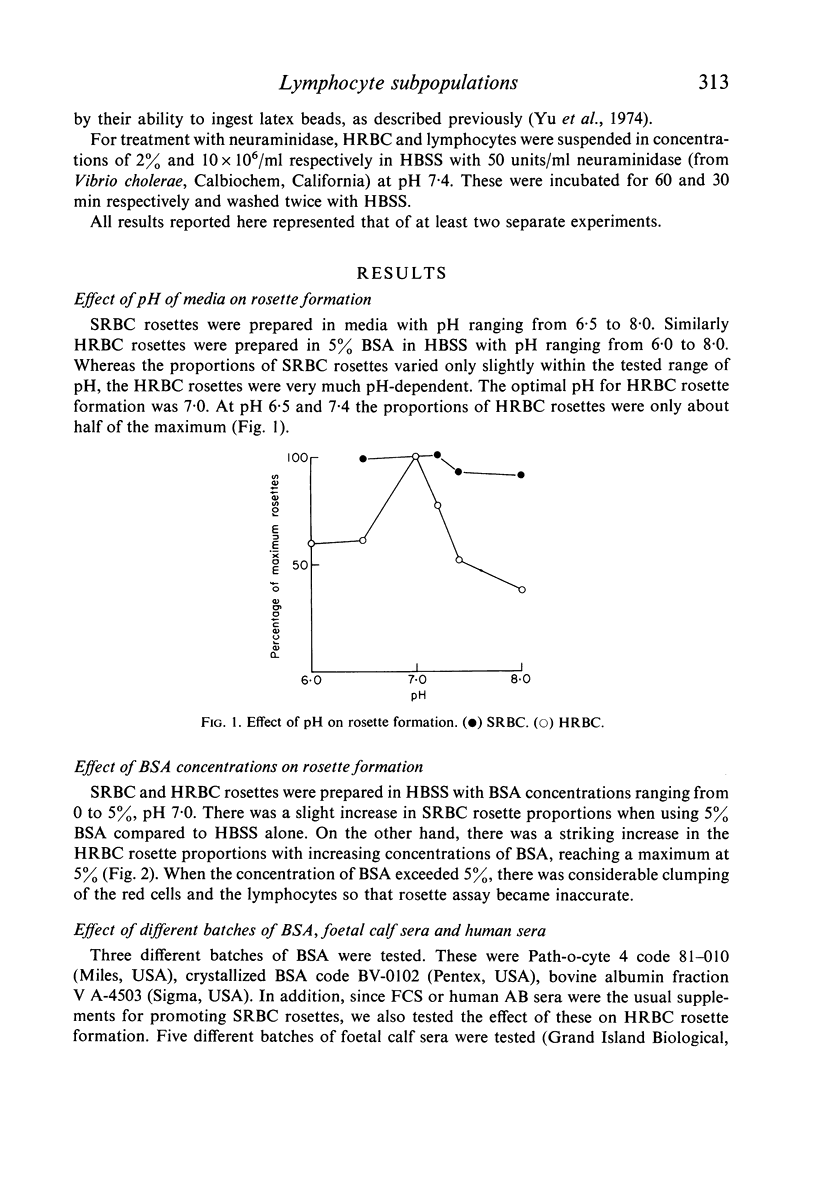
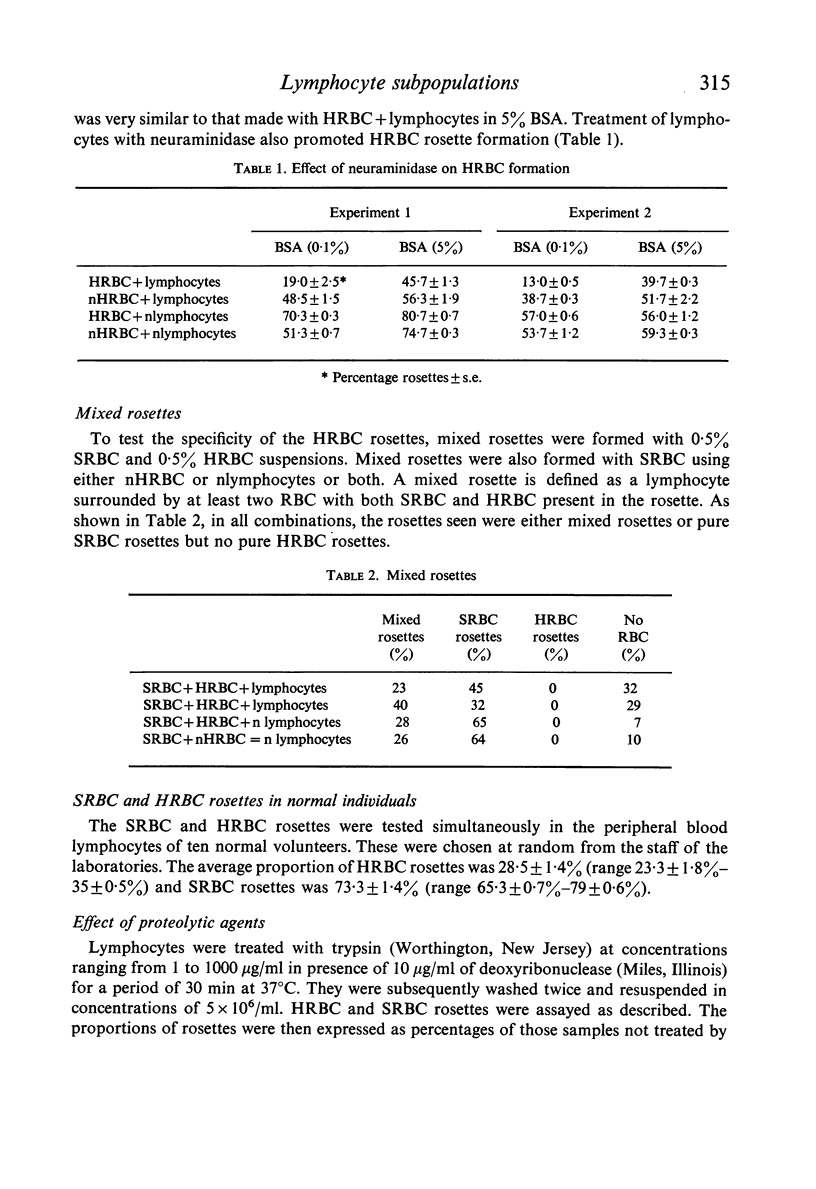
Abstract
Human red blood cells (HRBC) even without prior neuraminidase treatment, could form rosettes with human peripheral blood lymphocytes in vitro. The optimum conditions for forming these rosettes were a pH of 7-0 and a medium with 5% bovine serum albumin (BSA). Rosette proportions became much less at a different pH or using lower concentrations of BSA, or replacing BSA with foetal calf sera (FCS) or human sera. Rosette formation was also promoted by prior treatment of HRBC or lymphocytes with neuraminidase. Mixed rosettes of HRBC and sheep red blood cells (SRBC) showed that HRBC receptors were detectable only on lymphocytes that possessed SRBC receptors, suggesting that HRBC rosette-forming cells were probably thymus-derived (T) cells. Next, the properties of human red blood cell (HRBC) and sheep red blood cell (SRBC) rosette-forming cells were investigated by comparing the ability of human peripheral blood lymphocytes to form these two types of rosettes after treatment with various inhibitory reagents. HRBC rosettes were relatively more resistant to inhibition with: (1) proteolytic agents, such as trypsin, alpha-chymotrypsin and pronase; (2) anti-thymocyte serum (ATS); (3) metabolic inhibitors, such as sodium azide and 2,4-dinitrophenol (DNP); (4) cytochalasin B. On further incubation after trypsinization, the lymphocytes recovered some ability to form SRBC rosettes, but continued to lose more of their capability to form HRBC rosettes. All these results were regarded as circumstantial evidence that the HRBC rosettes might represent a subpopulation of human T lymphocytes.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F. Evaluation of T-cells and thymic serum factors in man using the rosette technique. Transplant Rev. 1973;16(0):196–217. doi: 10.1111/j.1600-065x.1973.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Baxley G., Bishop G. B., Cooper A. G., Wortis H. H. Rosetting of human red blood cells to thymocytes and thymus-derived cells. Clin Exp Immunol. 1973 Nov;15(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Skutelsky E., Kunkel H. G. Sheep red cell binding to human lymphocytes treated with neuraminidase; enhancement of T cell binding and identification of a subpopulation of B cells. J Exp Med. 1973 Jun 1;137(6):1532–1537. doi: 10.1084/jem.137.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwich Z., Kunkel H. G. Specific properties of human B and T lymphocytes and alterations in disease. Transplant Rev. 1973;16:29–50. doi: 10.1111/j.1600-065x.1973.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Brain P., Gordon J. Rosette formation by peripheral lymphocytes. II. Inhibition of the phenomenon. Clin Exp Immunol. 1971 Mar;8(3):441–449. [PMC free article] [PubMed] [Google Scholar]
- Cantor H. T cells and the immune response. Prog Biophys Mol Biol. 1972;25:73–82. doi: 10.1016/0079-6107(72)90016-8. [DOI] [PubMed] [Google Scholar]
- Chien S., Jan K. M. Red cell aggregation by macromolecules: roles of surface adsorption and electrostatic repulsion. J Supramol Struct. 1973;1(4):385–409. doi: 10.1002/jss.400010418. [DOI] [PubMed] [Google Scholar]
- Feizi T., Wernet P., Kunkel H. G., Douglas S. D. Lymphocytes forming red cell rosettes in the cold in patients with chronic cold agglutinin disease. Blood. 1973 Nov;42(5):753–762. [PubMed] [Google Scholar]
- Henney C. S., Bubbers J. E. Antigen-T lymphocyte interactions: inhibition by cytochalasin B. J Immunol. 1973 Jul;111(1):85–90. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey J. H., Hom D. J., Buttrick P. Human T lymphocyte receptors for sheep erythrocytes: conditions for binding including inhibition by cytochalasin B. J Immunol. 1974 Feb;112(2):862–865. [PubMed] [Google Scholar]
- Raff M. C. T and B lymphocytes and immune responses. Nature. 1973 Mar 2;242(5392):19–23. doi: 10.1038/242019a0. [DOI] [PubMed] [Google Scholar]
- SEAMAN G. V., HEARD D. H. The surface of the washed human erythrocyte as a polyanion. J Gen Physiol. 1960 Nov;44:251–268. doi: 10.1085/jgp.44.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. E., Mehrishi J. N. Surface properties of normal human circulating small lymphocytes and lymphocytes in chronic lymphocytic leukaemia: separation, adhesiveness and electrokinetic properties. Eur J Cancer. 1969 May;5(2):195–206. doi: 10.1016/0014-2964(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Watson J., Epstein R. The role of humoral factors in the initiation of in vitro primary immune responses. I. Effects of deficient fetal bovine serum. J Immunol. 1973 Jan;110(1):31–42. [PubMed] [Google Scholar]
- Yu D. T. Human lymphocyte receptor movement induced by sheep erythrocyte binding: effect of temperature and neuraminidase treatment. Cell Immunol. 1974 Nov;14(2):313–320. doi: 10.1016/0008-8749(74)90215-9. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Peter J. B., Paulus H. E., Nies K. M. Human lymphocyte subpopulations. Study of T and B cells and their density distribution. Clin Immunol Immunopathol. 1974 Apr;2(3):333–342. doi: 10.1016/0090-1229(74)90051-8. [DOI] [PubMed] [Google Scholar]


