Abstract
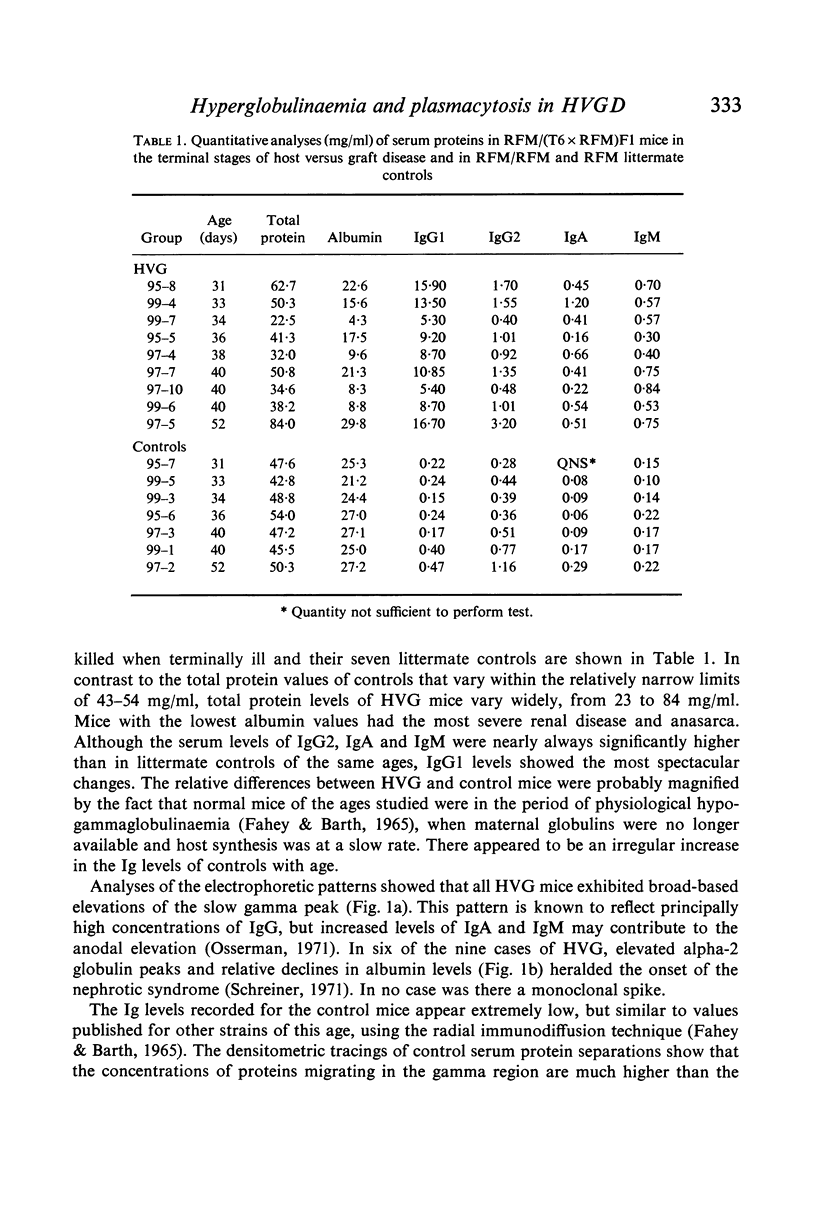
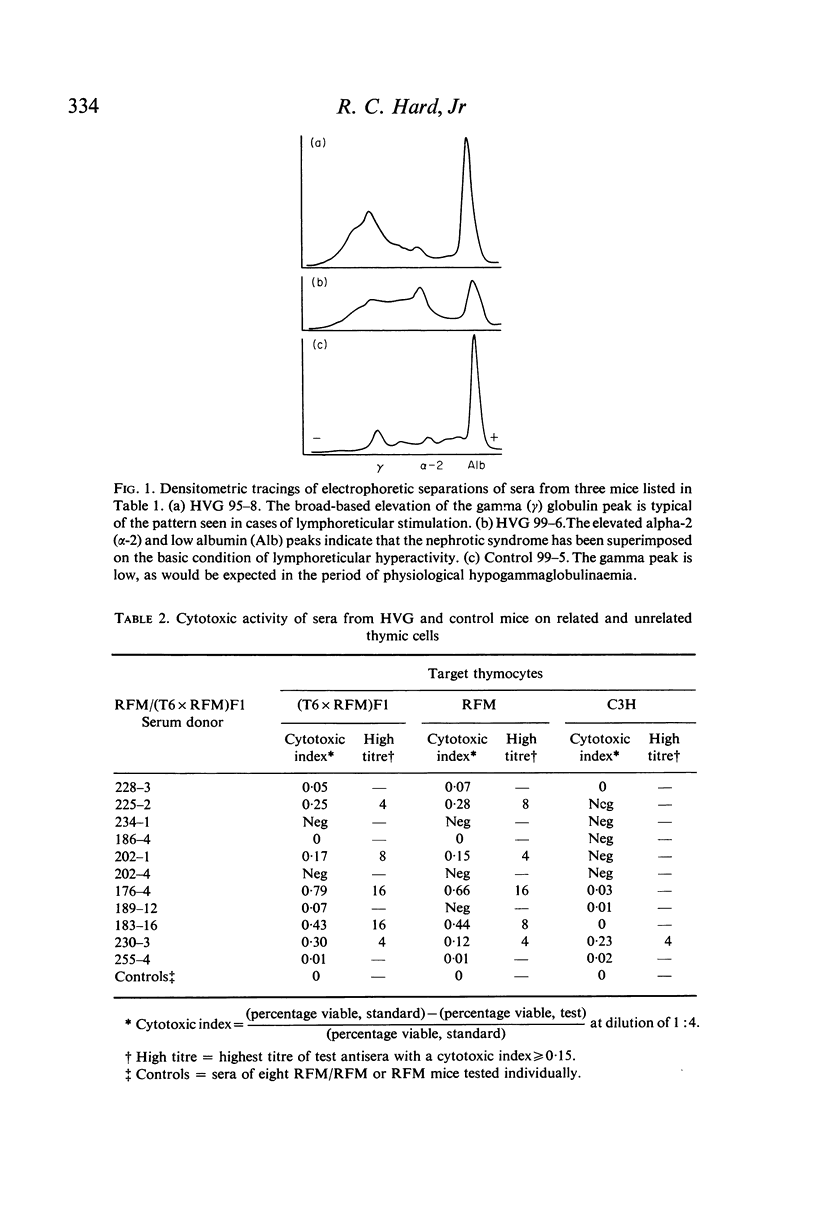
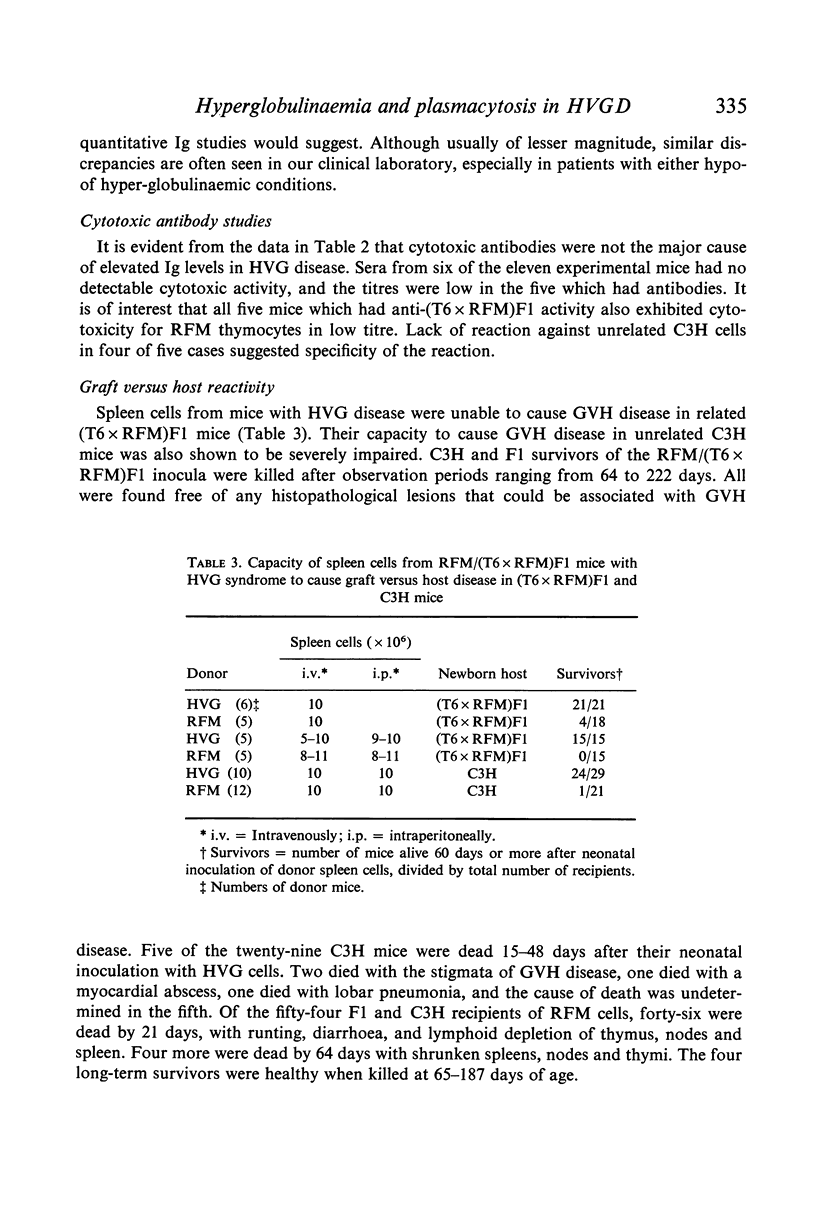
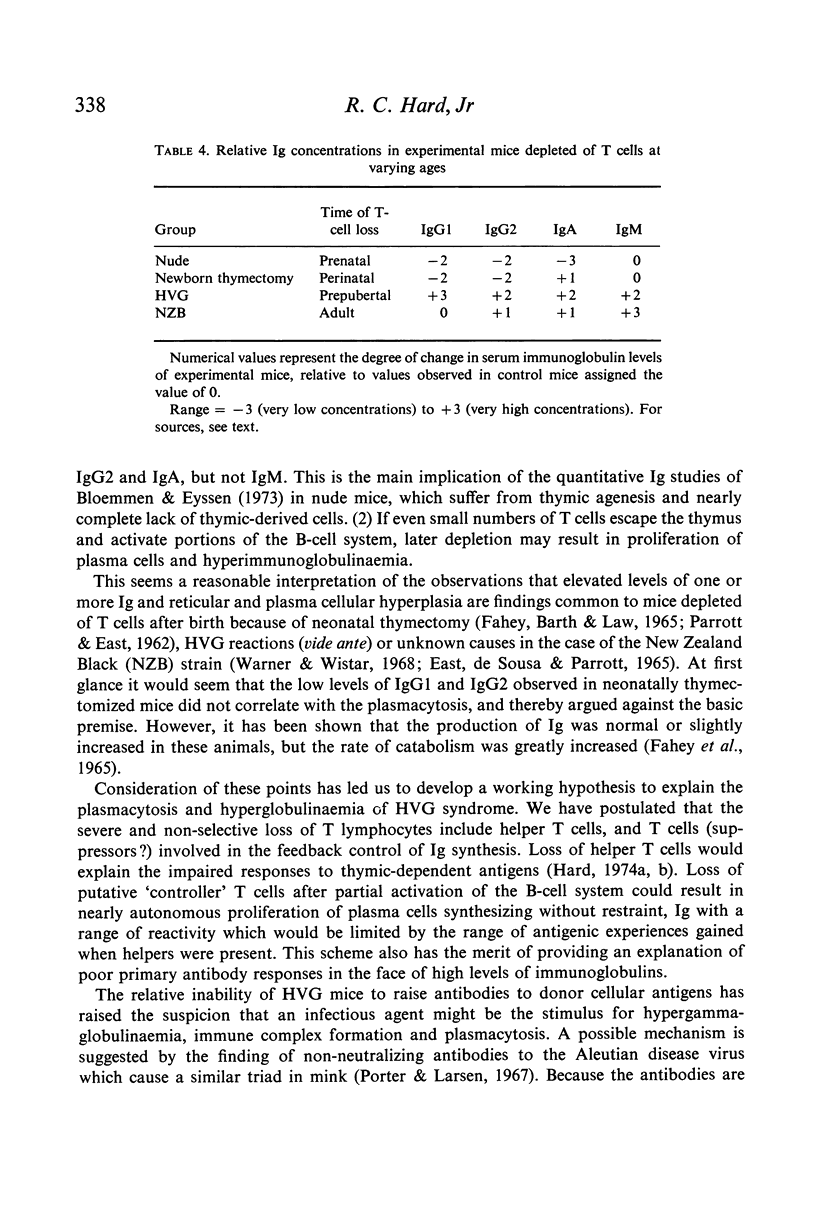
Host versus graft (HVG) syndrome may be induced in parental strain mice by perinatal inoculations of F1 hybrid spleen cells. The principal manifestations of the disease include thrombocytopaenia, intravascular fibrin deposits, intestinal haemorrhage, hepatic infarcts, lymphosplenomegaly and renal disease. Immune complexes have been shown to be the cause of the renal lesions, and have been implicated as the triggers for disseminated intravascular coagulation. In the present studies of RFM mice perinatally inoculated with (T6 x RFM)F1 spleen cells (RFM/(T6 x RFM)F1 mice), quantitative determinations of serum immunoglobulins (Ig) revealed marked elevations of IgG1, IgG2, IgA and IgM. Electrophoretic analyses revealed the polyclonal pattern which typically follows chronic antigenic stimulation. However, IgG1 levels which reached 29 to 72 times control values suggested disruption of homeostatic mechanisms which control circulating Ig levels. Because antibody responses to histocompatibility antigens were present only occasionally, and then in low titre, it seemed unlikely these antigens were the principal causes of hypergammaglobulinaemia and plasmacytosis. Morphological studies indicated that the elevated levels of Ig seen in end-stage HVG syndrome correlated well with marked plasmacytosis, the third morphological finding in a sequence that included the precocious development of germinal centres and subsequent depletion of thymic-dependent (T) lymphocytes. The fact that spleen cells from RFM/(T6 x RFM)F1 mice were severely impaired in their capacity to cause graft versus host disease in related (T6 x RFM)F1 and unrelated C3H mice provided strong evidence that the HVG reaction resulted in T-cell depletion, rather than specific immunoincompetence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth W. F., McLaughlin C. L., Fahey J. L. The immunoglobulins of mice. VI. Response to immunization. J Immunol. 1965 Nov;95(5):781–790. [PubMed] [Google Scholar]
- Bloemmen J., Eyssen H. Immunoglobulin levels of sera of genetically thymusless (nude) mice. Eur J Immunol. 1973 Feb;3(2):117–118. doi: 10.1002/eji.1830030213. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Coe J. E. 7Sgamma-1 and 7Sgamma-2 antibody response in the mouse. I. Influence of strain, antigen and adjuvant. J Immunol. 1966 Apr;96(4):744–753. [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- East J., de Sousa M. A., Parrott D. M. Immunopathology of New Zealand black (NZB) mice. Transplantation. 1965 Nov;3(6):711–729. doi: 10.1097/00007890-196511000-00003. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., BARTH W. F. THE IMMUNOGLOBULINS OF MICE. 4. SERUM IMMUNOGLOBULIN CHANGES FOLLOWING BIRTH. Proc Soc Exp Biol Med. 1965 Mar;118:596–600. doi: 10.3181/00379727-118-29914. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., ROBINSON A. G. FACTORS CONTROLLING SERUM GAMMA-GLOBULIN CONCENTRATION. J Exp Med. 1963 Nov 1;118:845–868. doi: 10.1084/jem.118.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK M. A., QUINN V. A. Antibody production in inbred strains of mice. J Immunol. 1953 Jan;70(1):61–67. [PubMed] [Google Scholar]
- Fahey J. L., Barth W. F., Law L. W. Normal immunoglobulins and antibody response in neonatally thymectomized mice. J Natl Cancer Inst. 1965 Oct;35(4):663–678. [PubMed] [Google Scholar]
- Fudenberg H. H. Genetically determined immune deficiency as the predisposing cause of "autoimmunity" and lymphoid neoplasia. Am J Med. 1971 Sep;51(3):295–298. doi: 10.1016/0002-9343(71)90263-4. [DOI] [PubMed] [Google Scholar]
- Grishman E., Porush J. C., Rosen S. M., Churg J. Lupus nephritis with organized deposits in the kidneys. Lab Invest. 1967 May;16(5):717–725. [PubMed] [Google Scholar]
- Hard R. C., Jr Correlation of immunological function with splenic histopathology during the preclinical stages of host-versus-graft syndrome in parent-F1 mouse chimeral. Transplantation. 1974 Jun;17(6):613–623. doi: 10.1097/00007890-197406000-00011. [DOI] [PubMed] [Google Scholar]
- Hard R. C., Jr, Kullgren B. Etiology, pathogenesis, and prevention of a fatal host-versus-graft syndrome in parent-F1 mouse chimeras. Am J Pathol. 1970 May;59(2):203–224. [PMC free article] [PubMed] [Google Scholar]
- Hard R. C., Jr, Moncure C. W., Still W. J. Renal lesions with organized deposits and lipid as part of the host versus graft syndrome in parent-F 1 mouse chimeras. Lab Invest. 1973 Apr;28(4):468–476. [PubMed] [Google Scholar]
- Hard R. C., Jr, Still W. J. Intravascular fibrin deposits, hepatic infarcts and thrombocytopenia in parent/F mouse chimeras with host-versus-graft syndrome. Am J Pathol. 1975 Apr;79(1):131–146. [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Chan E. L., Ravitch M. M., Riblet R. J., Herzenberg L. A. Active suppression of immunoglobulin allotype synthesis. 3. Identification of T cells as responsible for suppression by cells from spleen, thymus, lymph node, and bone marrow. J Exp Med. 1973 Jun 1;137(6):1311–1324. doi: 10.1084/jem.137.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Kaehler S. L. Antibody to leukemia virus: widespread occurrence in inbred mice. Science. 1974 Sep 6;185(4154):869–871. doi: 10.1126/science.185.4154.869. [DOI] [PubMed] [Google Scholar]
- PARROTT D. M., EAST J. Role of the thymus in neonatal life. Nature. 1962 Jul 28;195:347–348. doi: 10.1038/195347a0. [DOI] [PubMed] [Google Scholar]
- POPP D. M., AMOS D. B. AN H-2 ANALYSIS OF STRAIN RFM/UN MICE. Transplantation. 1965 Jul;3:501–508. doi: 10.1097/00007890-196507000-00005. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. A., FITCH F. W. HOMEOSTASIS OF ANTIBODY FORMATION IN THE ADULT RAT. J Exp Med. 1964 Dec 1;120:987–1005. doi: 10.1084/jem.120.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. T and B lymphocytes and immune responses. Nature. 1973 Mar 2;242(5392):19–23. doi: 10.1038/242019a0. [DOI] [PubMed] [Google Scholar]
- Ramseier H., Lindenmann J. Aliotypic antibodies. Transplant Rev. 1972;10:57–96. doi: 10.1111/j.1600-065x.1972.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Simpson E., O'Hopp S., Harrison M., Mosier D., Melief K., Cantor H. Immunological disease induced by injecting F1 lymphoid cells into certain parental strains. Immunology. 1974 Dec;27(6):989–1007. [PMC free article] [PubMed] [Google Scholar]
- TALAL N., HERMANN G., VAUXSTCYRC DE, GRABAR P. IMMUNOELECTROPHORETIC CHANGES IN MOUSE GAMMA-GLOBULIN AFTER INTRAPERITONEAL INJECTION OF BAYOL F. J Immunol. 1964 May;92:747–753. [PubMed] [Google Scholar]
- Upton A. C., Jenkins V. K., Walburg H. E., Jr, Tyndall R. L., Conklin J. W., Wald N. Observations on viral, chemical, and radiation-induced myeloid and lymphoid leukemias in RF mice. Natl Cancer Inst Monogr. 1966 Sep;22:329–347. [PubMed] [Google Scholar]
- Warner N. L., Wistar R., Jr Immunoglobulins in NZB-BL mice. I. Serum immunoglobulin levels and immunoglobulin class of erythrocyte autoantibody. J Exp Med. 1968 Jan 1;127(1):169–183. doi: 10.1084/jem.127.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]



