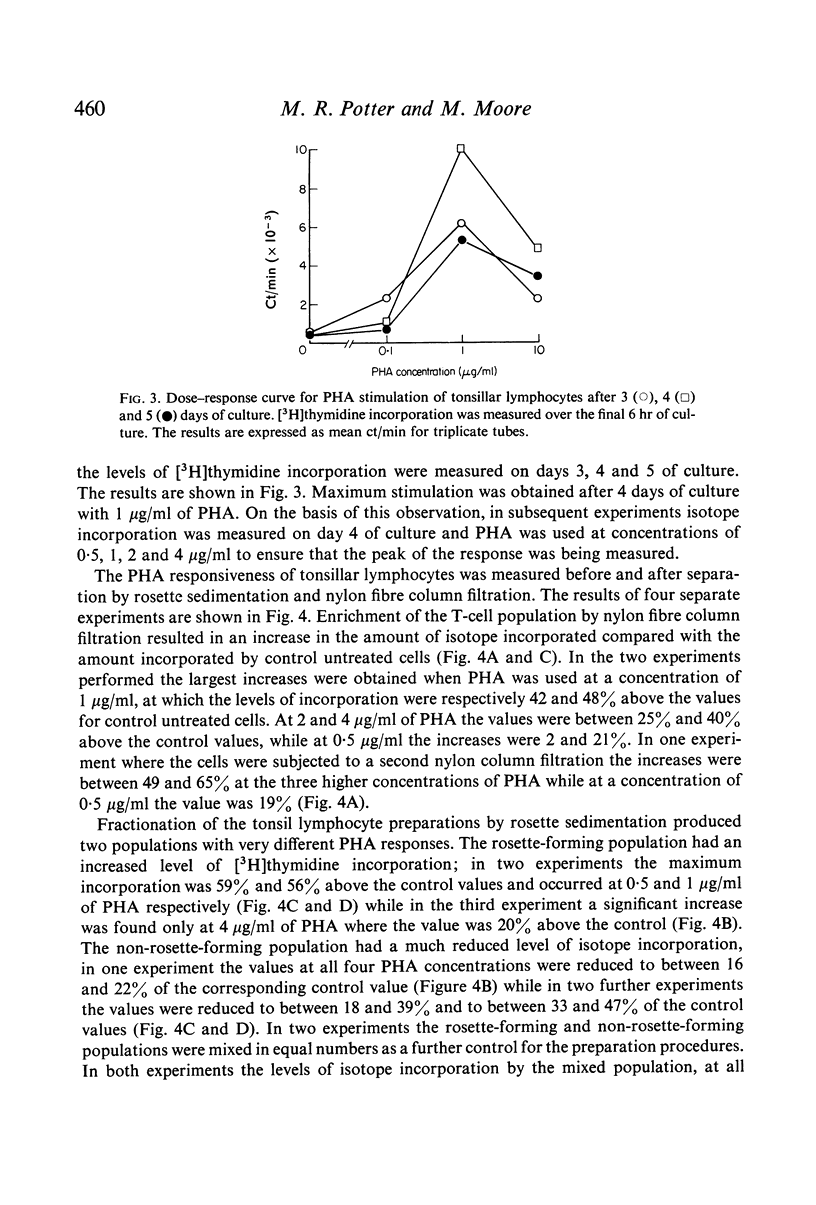
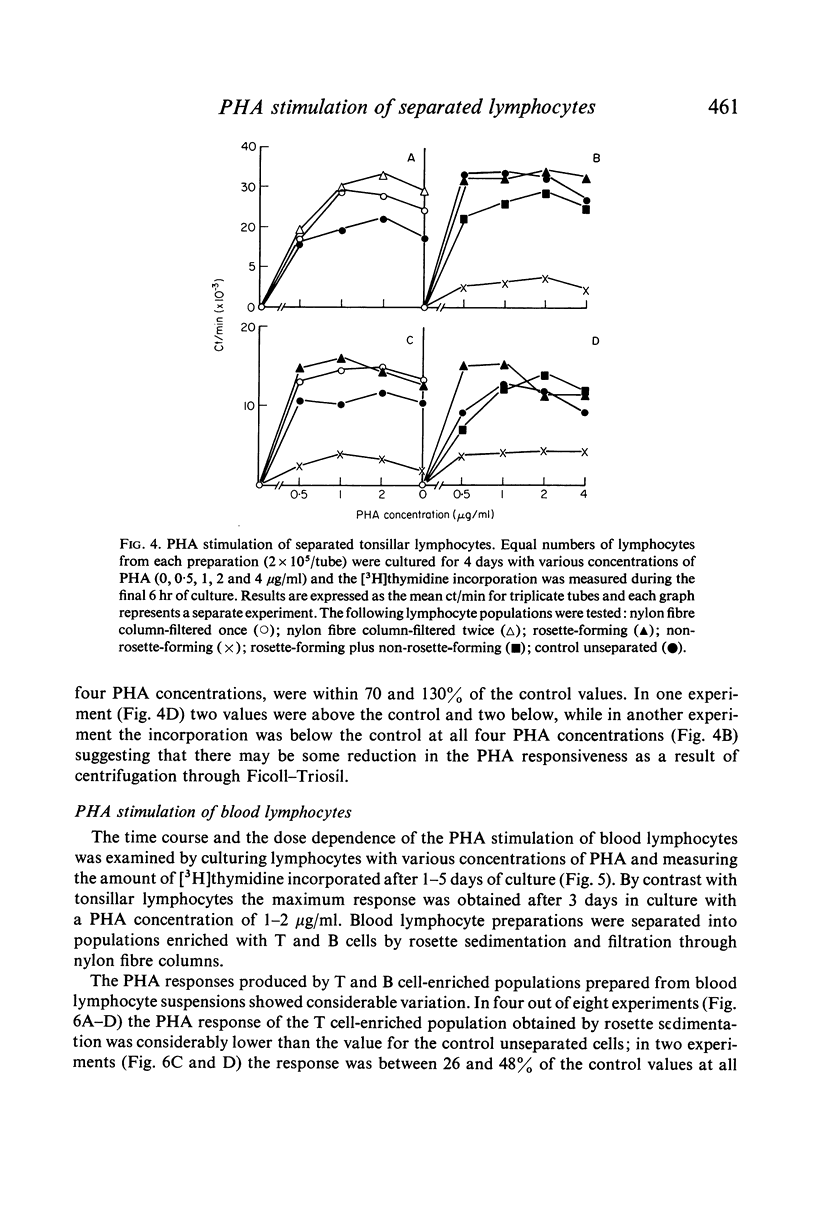
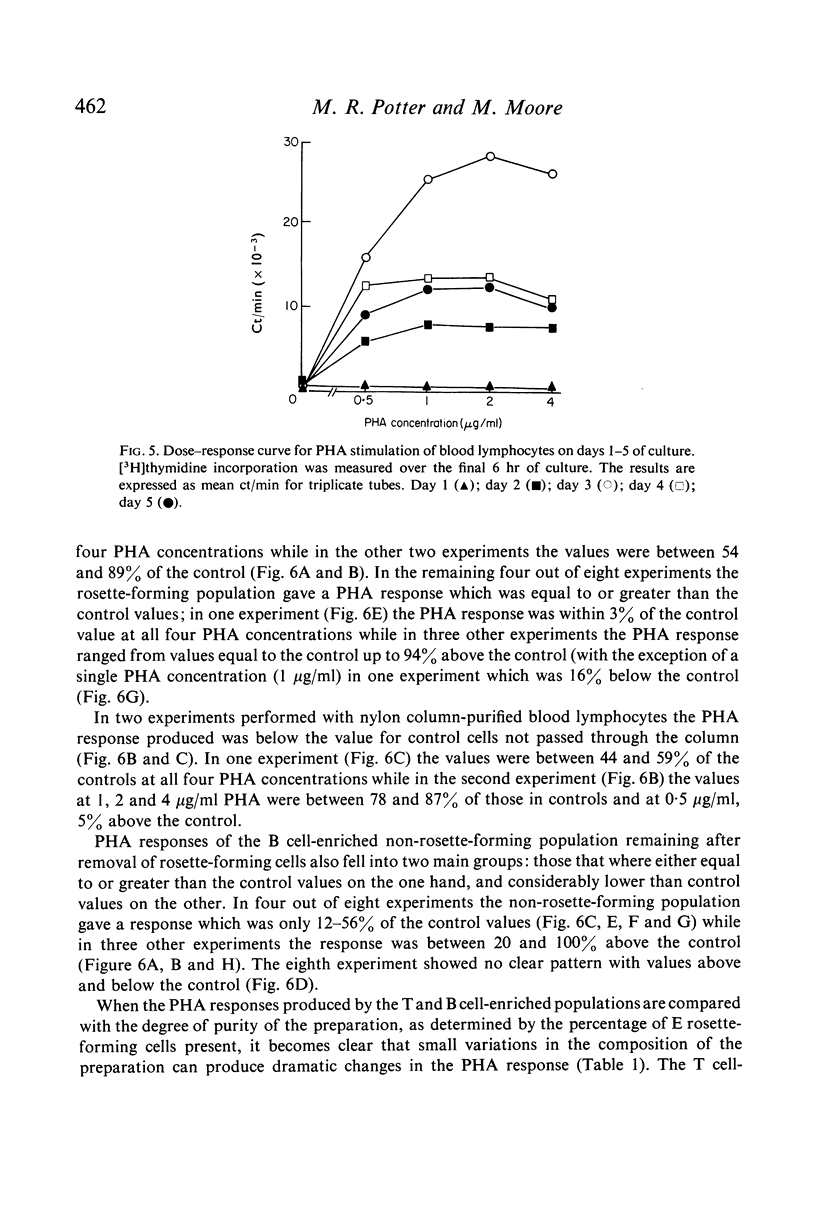
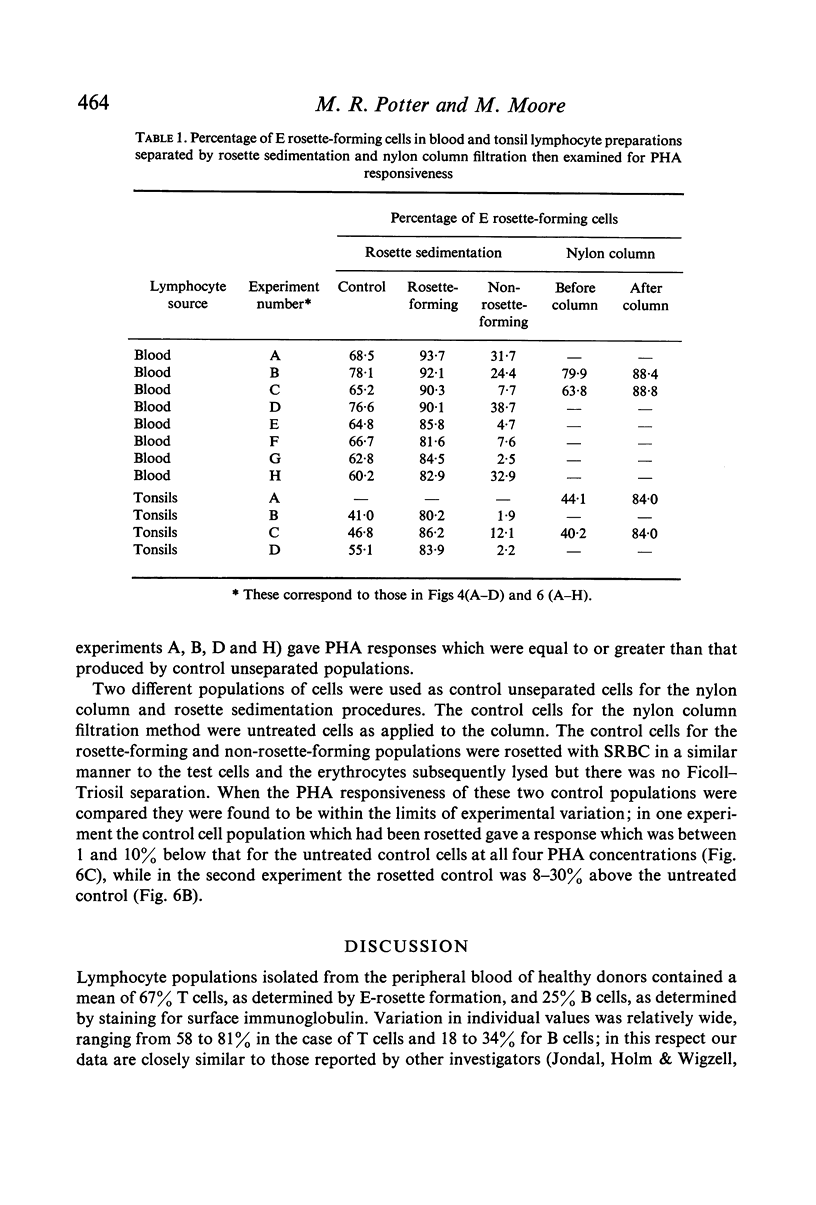
Abstract
Lymphocyte preparations from peripheral blood and tonsils were separated into populations enriched with T or B cells by formation of rosettes with SRBC and separation of the rosette-forming and non-rosette-forming populations. T cell-enriched populations were also prepared by nylon column filtration. Using these methods preparations were obtained which comprised 80--95% T or B lymphocytes as determined by E-rosette formation and surface immunoglobulin (Ig) staining. PHA responsiveness, measured by [3H]thymidine incorporation, varied between relatively wide limits and was critically dependent on the degree of separation obtained. Relatively pure B-cell populations (less than 12% T cells) from blood and tonsils gave low PHA responses while preparations from blood still containing 24--38% T cells gave responses equal to or even greater than those of unseparated controls (60--78% T cells). T cell-enriched populations (80--86% T cells) responded to an equal or greater degree than controls but more efficient separation (greater than 90% T cells) resulted in markedly reduced stimulation. There was thus no simple correlation between the degree of phytomitogen-induced transformation and the number of T cells present. It is concluded that the low response of relatively pure T-cell populations may be due to depletion of B cells or non-lymphoid cells (or both) during the separation procedures. These observations have implications for the use of PHA stimulation as a measure of T-cell activity in mixed populations such as those of human peripheral blood leucocytes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G., Greaves M. F. Enumeration of absolute numbers of T and B lymphocytes in human blood. Scand J Immunol. 1974;3(2):161–172. doi: 10.1111/j.1365-3083.1974.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Greaves M. F., Roitt I. M., Rose M. E. Effect of bursectomy and thymectomy on the responses of chicken peripheral blood lymphocytes to phytohaemagglutinin. Nature. 1968 Oct 19;220(5164):293–295. doi: 10.1038/220293a0. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G., Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974 Jul 1;140(1):1–18. doi: 10.1084/jem.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedfors E. Activation of peripheral T cells of sarcoidosis patients and healthy controls. Clin Exp Immunol. 1974 Nov;18(3):379–390. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Hudson L. Specific purification of lymphocyte populations on a digestible immunoabsorbent. J Immunol. 1973 Jan;110(1):313–319. [PubMed] [Google Scholar]
- Takiguchi T., Adler W. H., Smith R. T. Cellular recognition in vitro by mouse lymphocytes. Effects of neonatal thymectomy and thymus graft restoration on alloantigen and PHA stimulation of whole and gradient-separated subpopulations of spleen cells. J Exp Med. 1971 Jan 1;133(1):63–80. doi: 10.1084/jem.133.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]
- Yata J., Desgranges C., Tachibana T., de Thé G. Separation of human lymphocytes forming spontaneous rosettes with sheep erythrocytes. Biomedicine. 1973 Nov 20;19(11):475–478. [PubMed] [Google Scholar]
- Zeylemaker W. P., Roos M. T., Meyer C. J., Schellekens P. T., Eijsvoogel V. P. Separation of human lymphocyte subpopulations. I. Transformation characteristics of rosette-forming cells. Cell Immunol. 1974 Dec;14(3):346–358. doi: 10.1016/0008-8749(74)90184-1. [DOI] [PubMed] [Google Scholar]


