Abstract
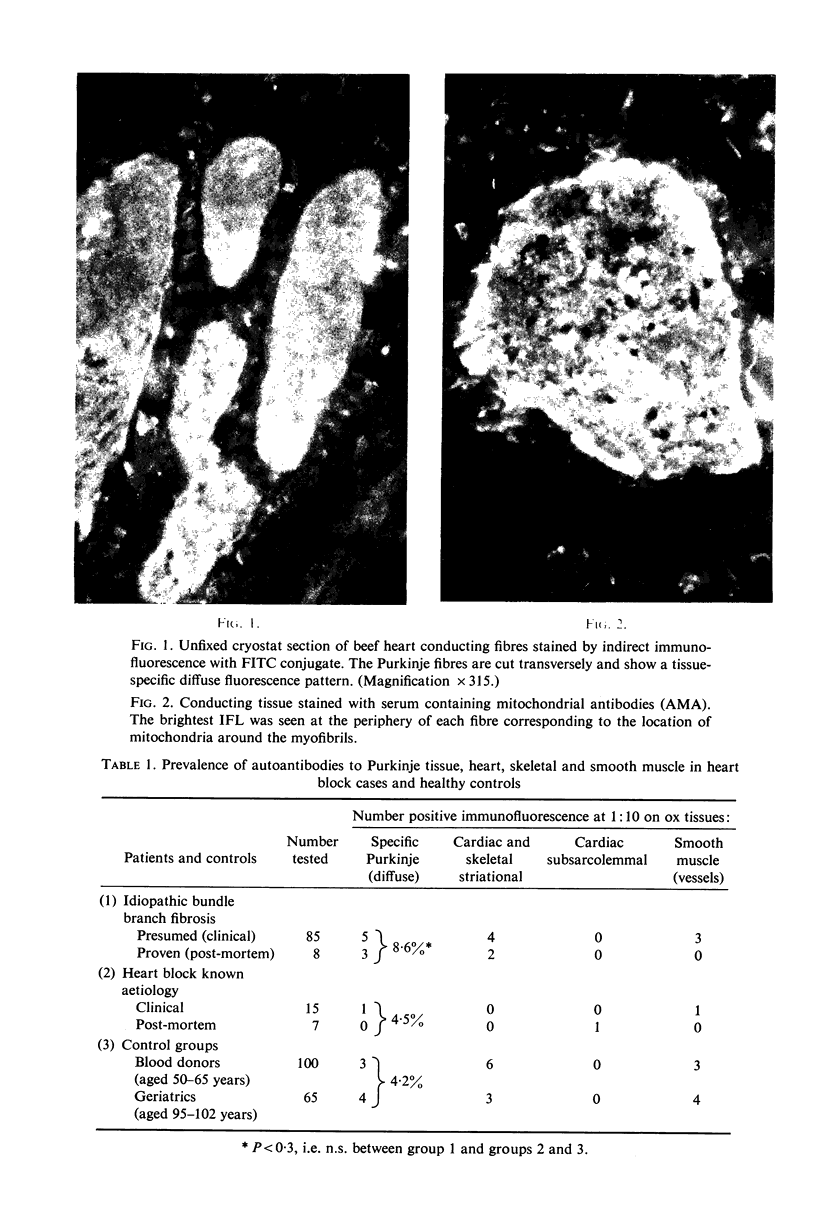
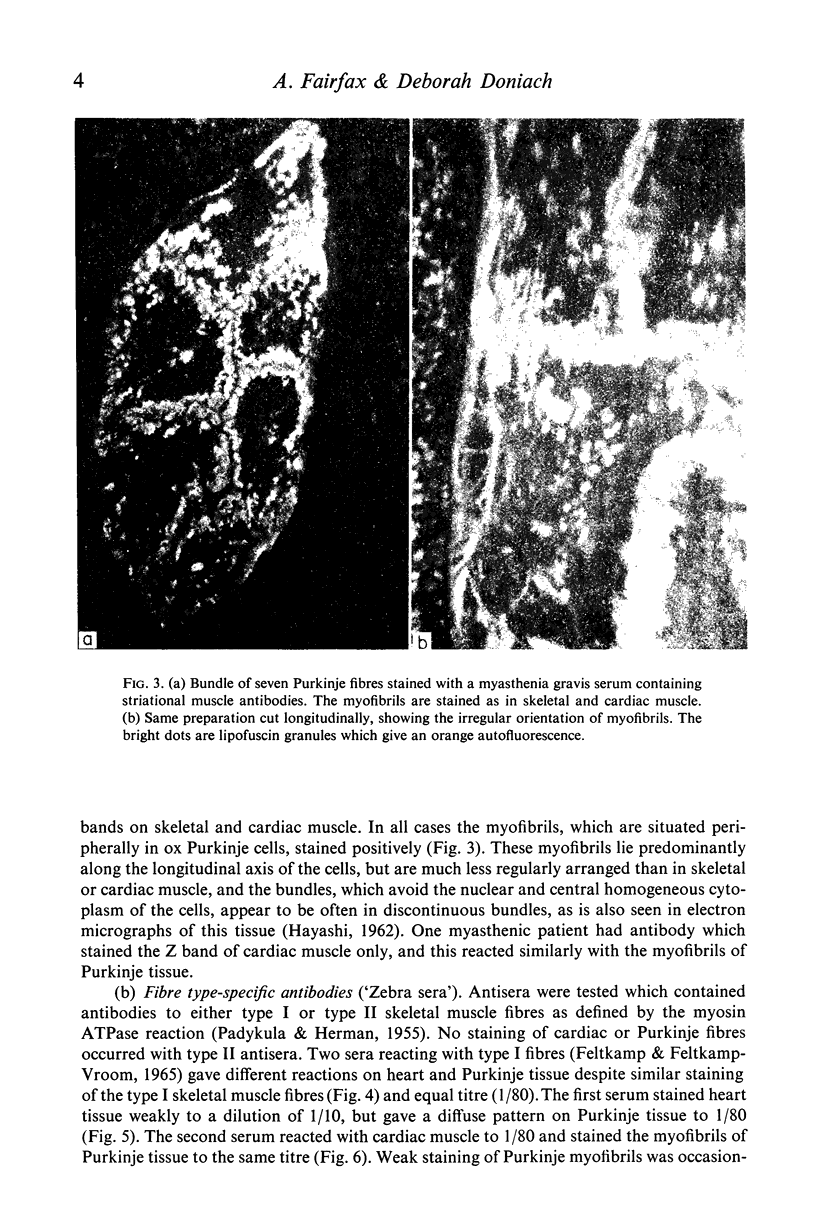
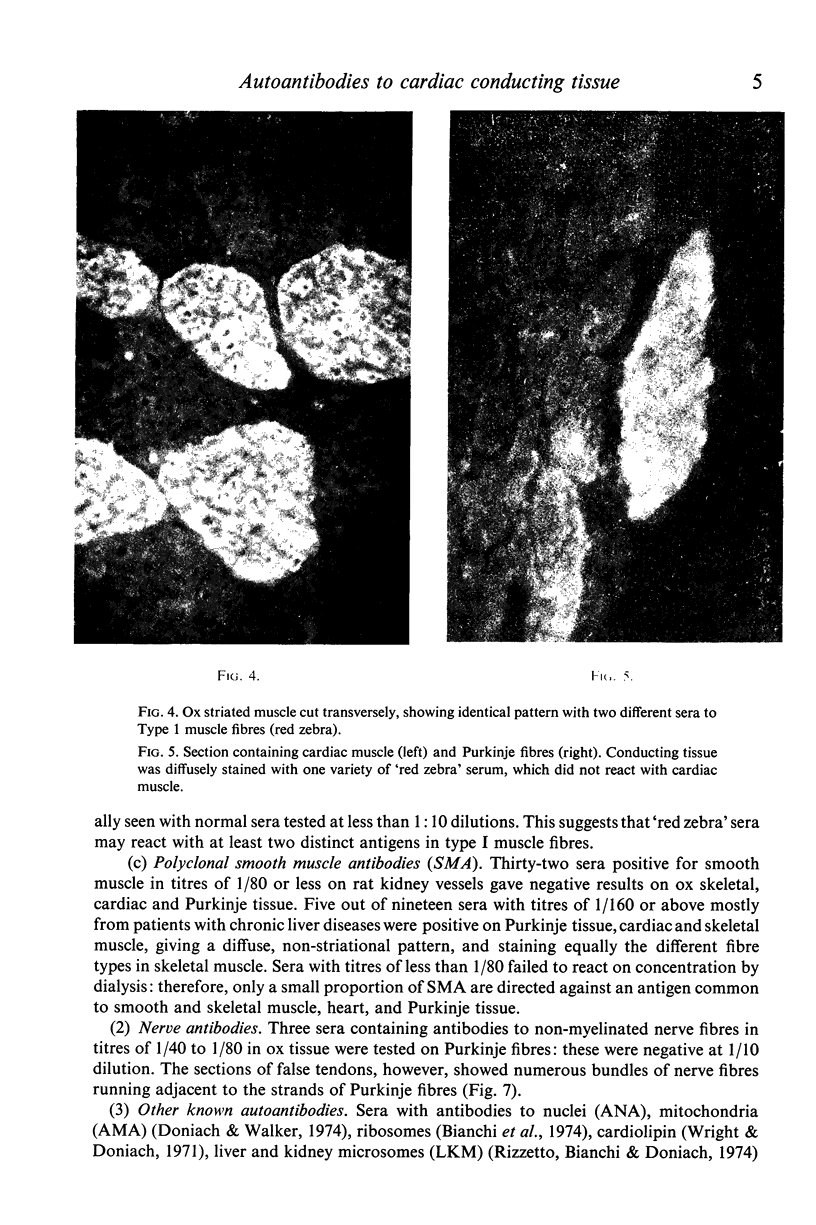
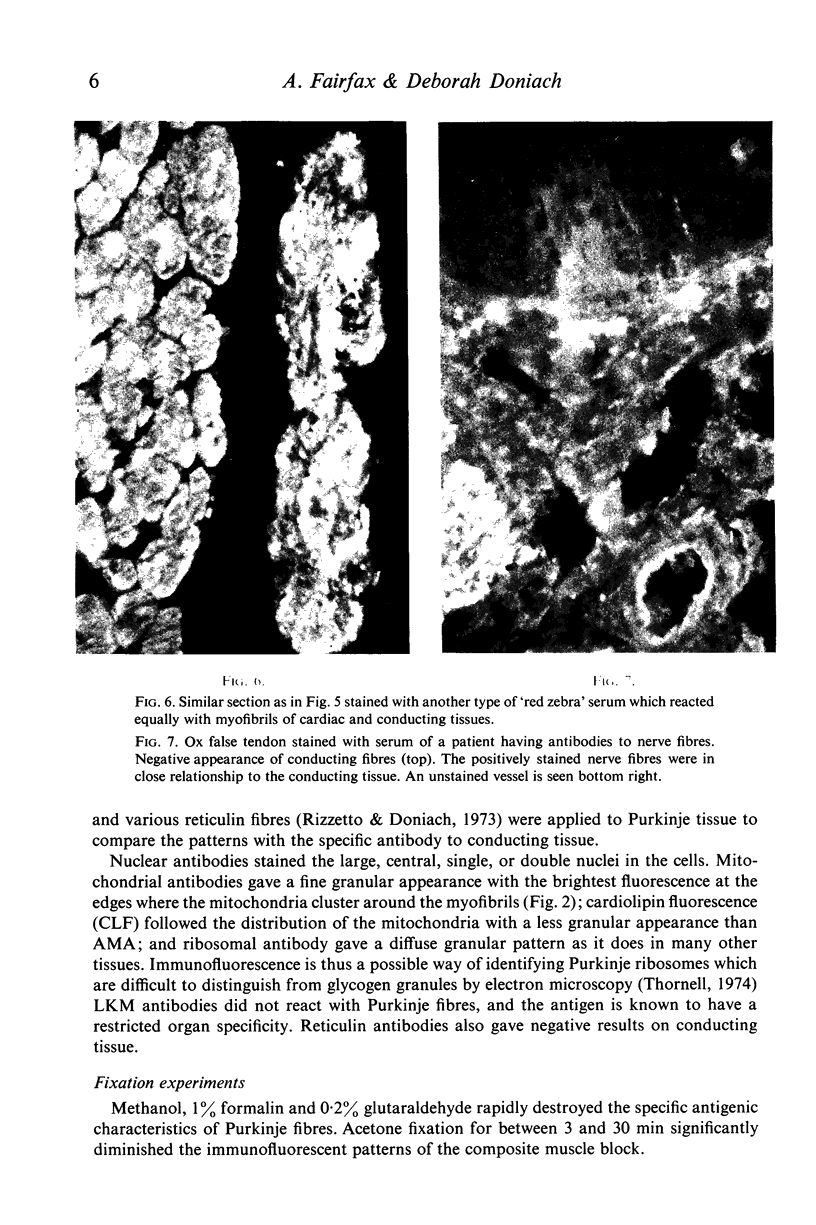
(1) A new antibody has been found by immunofluorescence which reacts with cardiac conducting tissue using ox heart false tendons. It was detected in eight out of ninety-three cases of idiopathic heart block (8-6%), in one out of twenty-two cases of secondary heart block (4-5%) and in seven of 165 normal controls (4.2%), in titres varying from 1:10 to 1:40. Previous authors had indicated that this tissue might contain unique antigens. (2) Sera reacting with type I fibres in skeletal muscle (red zebra) were found to be of two varieties, one of which stained conducting fibres diffusely while it gave minimal staining of cardiac muscle; the other reacting with myofibrils in Purkinje cells and heart. (3) Some sera with high titre smooth muscle antibodies (SMA) reacted with conducting tissue together with skeletal and cardiac muscle, suggesting that the four tissues have at least one antigen in common. (4) Known non-organ specific antibodies behaved as expected on beef conducting fibres: striational fluorescence of myasthenia gravis sera reacted with the same patterns on Purkinje myofibrils; AMA and ANA produced IFL in expected locations; ribosomal antibodies reacted strongly, while LKM and reticulin antibodies showed no reactivity. (5) Although the incidence of specific Purkinje fibre antibodies was not significantly raised in idiopathic heart block, the clinical associations suggest that some cases might be related to autoimmunity possibly involving cell-mediated mechanisms as in polymyositis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi F. B., Rizzetto M., Penfold P., Swana G. T., Doniach D. Ultrastructural localization and characterization of a ribosomal antibody detected by immunofluorescence in systemic lupus erythematosus. Clin Exp Immunol. 1974 Aug;17(4):629–636. [PMC free article] [PubMed] [Google Scholar]
- Bieber C. P., Stinson E. B., Shumway N. E. Pathology of the conduction system in cardiac rejection. Circulation. 1969 May;39(5):567–575. doi: 10.1161/01.cir.39.5.567. [DOI] [PubMed] [Google Scholar]
- CARBONELL L. M. Esterases of the conductive system of the heart. J Histochem Cytochem. 1956 Mar;4(2):87–95. doi: 10.1177/4.2.87. [DOI] [PubMed] [Google Scholar]
- Campbell A., Caird F. I., Jackson T. F. Prevalence of abnormalities of electrocardiogram in old people. Br Heart J. 1974 Oct;36(10):1005–1011. doi: 10.1136/hrt.36.10.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R. L., Eghtedari A., Holborow E. J. Antibodies to skeletal muscle demonstrated by immunofluorescence in experimental autoallergic myositis. Clin Exp Immunol. 1971 Sep;9(3):329–337. [PMC free article] [PubMed] [Google Scholar]
- Doniach D., Walker G. Mitochondrial antibodies (AMA). Gut. 1974 Aug;15(8):664–668. doi: 10.1136/gut.15.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMART E. W., HELANDER E. Distribution of muscle protein in the fibers of the conduction system of the beef heart. Arch Pathol. 1960 Dec;70:730–739. [PubMed] [Google Scholar]
- Eraut C. D., Evans R. C., Shaw D. B. Heart block in Devonshire. Br Heart J. 1973 May;35(5):557–557. [PubMed] [Google Scholar]
- Feltkamp T. E., Feltkamp-Vroom T. M. Antibodies against the various types of skeletal muscle fibres. Immunology. 1965 Sep;9(3):275–279. [PMC free article] [PubMed] [Google Scholar]
- Friedman I., Laufer A., Ron N., Davies A. M. Experimental myocarditis: in vitro and in vivo studies of lymphocytes sensitized to heart extracts and group A streptococci. Immunology. 1971 Feb;20(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- HAYASHI K. An electron microscope study on the conduction system of the cow heart. Jpn Circ J. 1962 Oct;26:765–842. doi: 10.1253/jcj.26.765. [DOI] [PubMed] [Google Scholar]
- HELANDER E., EMMART E. W. Localization of myosin in the conduction bundle of beef heart. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:838–842. doi: 10.3181/00379727-101-25114. [DOI] [PubMed] [Google Scholar]
- Helander E. Studies of chemical components of the conducting system of the heart. II. The water soluble proteins. Cardiologia. 1965;47(3):146–157. doi: 10.1159/000168386. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H., Frengley J. D. Autoimmunity to the heart in cardiac disease. Current concepts of the relation of autoimmunity to rheumatic fever, postcardiotomyand postinfarction syndromes and cardiomyopathies. Am J Cardiol. 1969 Oct;24(4):459–473. doi: 10.1016/0002-9149(69)90489-5. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Bianchi F. B., Doniach D. Characterization of the microsomal antigen related to a subclass of active chronic hepatitis. Immunology. 1974 Mar;26(3):589–601. [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Doniach D. Types of 'reticulin' antibodies detected in human sera by immunofluorescence. J Clin Pathol. 1973 Nov;26(11):841–851. doi: 10.1136/jcp.26.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder J., Meijer A. E. Enzyme histochemical studies on the Purkinje fibres of canine, bovine and porcine hearts. Histochem J. 1970 Sep;2(5):395–409. doi: 10.1007/BF01004720. [DOI] [PubMed] [Google Scholar]
- Thornell L. E. Distinction of glycogen and ribosome particles in cow Purkinje fibers by enzymatic digestion en bloc and in sections. J Ultrastruct Res. 1974 May;47(2):153–168. doi: 10.1016/s0022-5320(74)80067-5. [DOI] [PubMed] [Google Scholar]
- Wright J. M., Doniach D. The significance of cardiolipin immunofluorescence (CLF). Proc R Soc Med. 1971 Apr;64(4):419–422. [PMC free article] [PubMed] [Google Scholar]


