Abstract
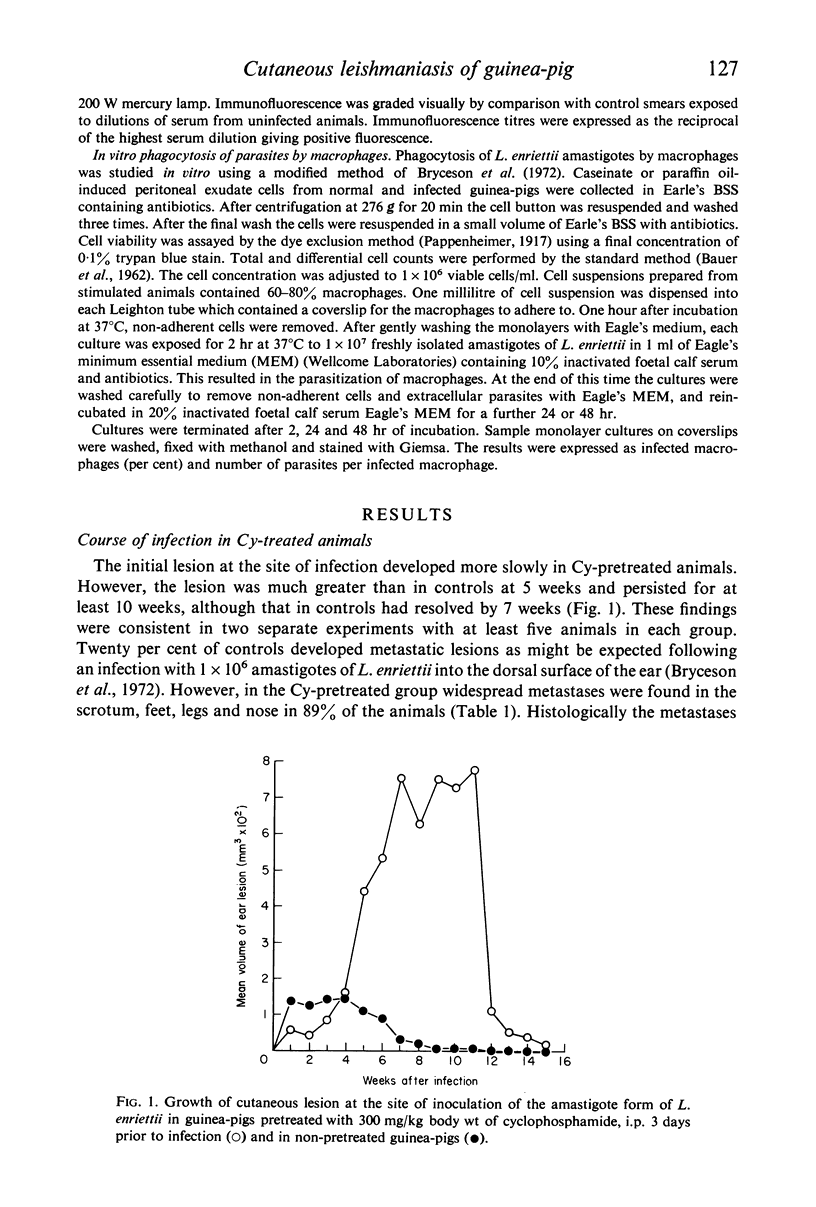
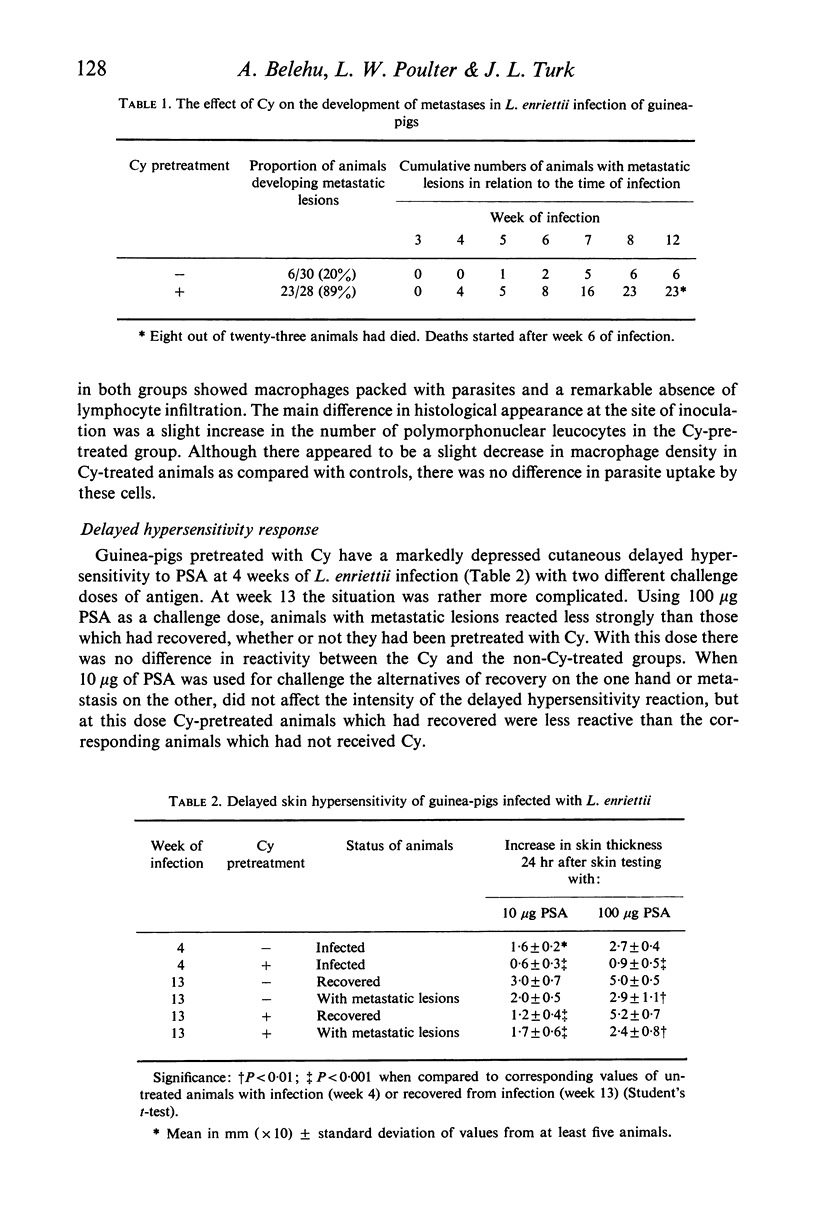
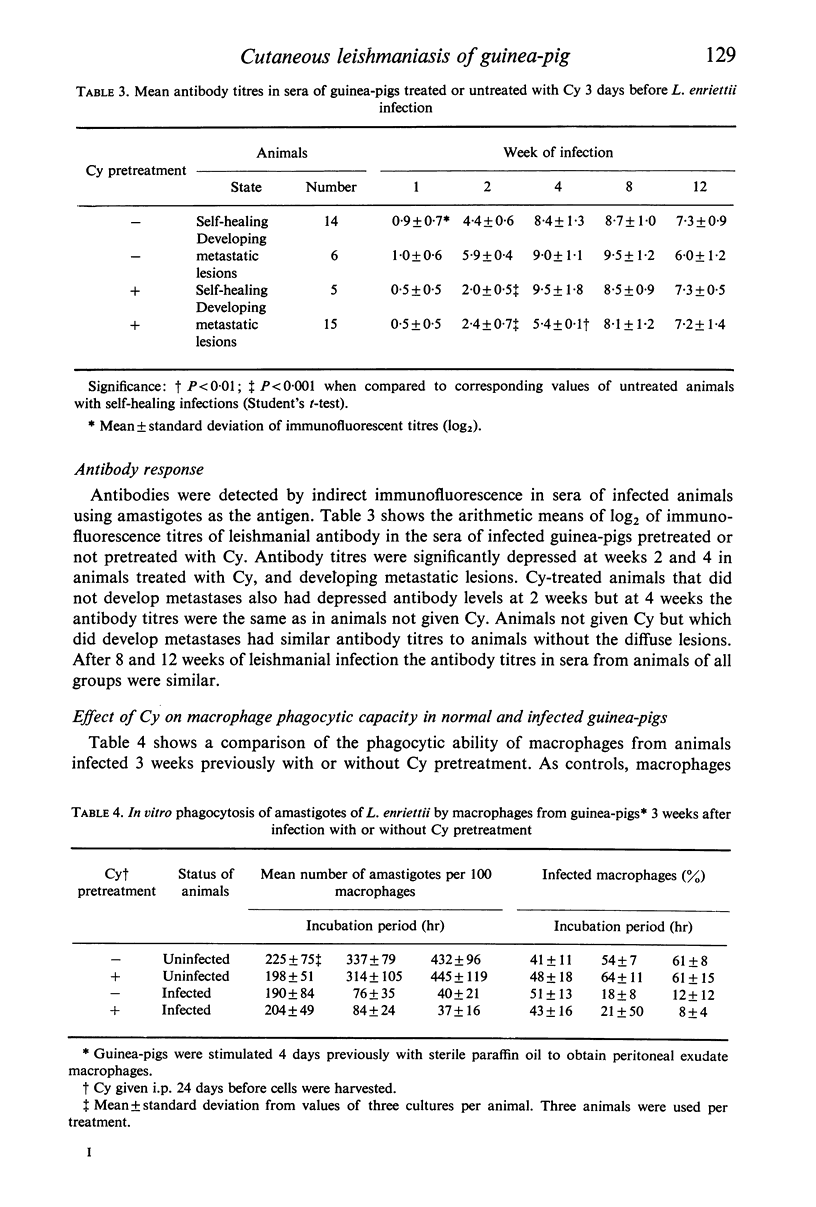
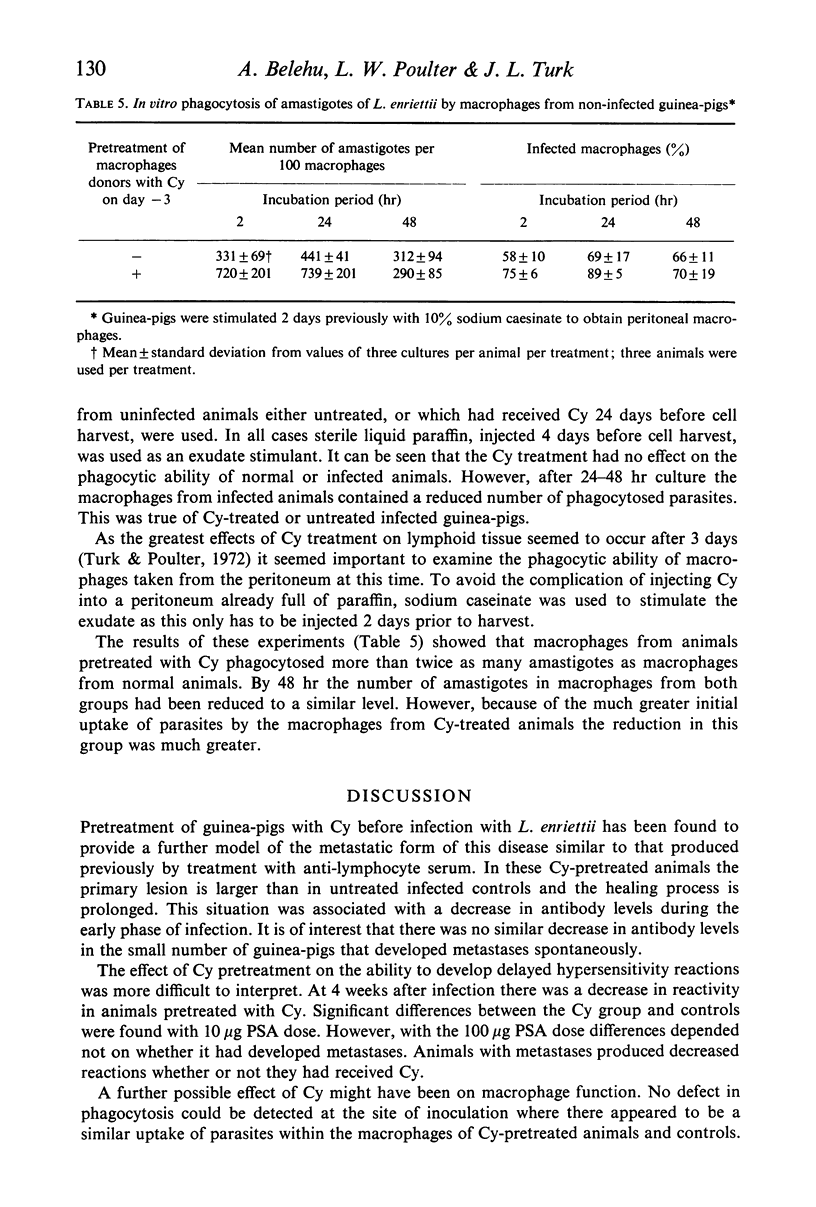
Pretreatment of guinea-pigs with cyclophosphamide (Cy) (300 mg/kg) 3 days before cutaneous infection with Leishmania enriettii caused an increased intensity of the lesion at the site of infection and an increase in the incidence of widespread metastases. Decreased levels of circulating antibody were found from the first to fourth week after infection. Decreased delayed type hypersensitivity could only be detected beyond 4 weeks. Peritoneal macrophages obtained form guinea-pigs 3 days after Cy pretreatment showed increase rather than decreased ability to phagocytose L. enriettii. Phagocytosis of L. enriettii by peritoneal macrophages obtained from guinea-pigs pretreated with Cy 24 days previously was normal. It is suggested that more attention should be taken of antibody levels during the early phase of infection and that control of infection could be due to a synergism between antibody and cell-mediated immunity. Upset in the balance by suppression of either function might lead to the development of widespread metastatic lesions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behin R., Mauel J., Biroum-Noerjasin, Rowe D. S. Mechanisms of protective immunity in experimental cutaneous leishmaniasis of the guinea-pig. II. Selective destruction of different Leishmania species in activated guinea-pig and mouse macrophages. Clin Exp Immunol. 1975 May;20(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Preston P. M., Bray R. S., Dumonde D. C. Experimental cutaneous leishmaniasis. II. Effects of immunosuppression and antigenic competition on the course of infection with Leishmania enriettii in the guinea-pig. Clin Exp Immunol. 1972 Feb;10(2):305–335. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Turk J. L. The effect of prolonged treatment with antilymphocyte serum on the course of infections with BCG and Leishmania enriettii in the guinea-pig. J Pathol. 1971 Jul;104(3):153–165. doi: 10.1002/path.1711040302. [DOI] [PubMed] [Google Scholar]
- Mauel J., Behin R., Biroum-Noerjasin, Rowe D. S. Mechanisms of protective immunity in experimental cutaneous leishmaniasis of the guinea-pig. I. Lack of effects of immune lymphocytes and of activated macrophages. Clin Exp Immunol. 1975 May;20(2):339–350. [PMC free article] [PubMed] [Google Scholar]
- Radwanski Z. K., Bryceson A. D., Preston P. M., Dumonde D. C. Immunoflorescence studies of Leishmania enriettii infection in the guinea pig. Trans R Soc Trop Med Hyg. 1974;68(2):124–132. doi: 10.1016/0035-9203(74)90185-0. [DOI] [PubMed] [Google Scholar]
- Revell P. A. Studies on the effect of cyclophosphamide on T and B lymphocytes in the blood, lymph nodes, and thymus of normal guinea pigs. Int Arch Allergy Appl Immunol. 1974;47(6):864–874. doi: 10.1159/000231277. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Behforouz N., Gettner S. Studies on anti-leishmania activity of immune rabbit serum. J Parasitol. 1970 Apr;56(2):350–353. [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Turk J. L., Parker D. Further studies on B-lymphocyte suppression in delayed hypersensitivity, indicating a possible mechanism for Jones-Mote hypersensitivity. Immunology. 1973 Apr;24(4):751–758. [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Parker D., Poulter L. W. Functional aspects of the selective depletion of lymphoid tissue by cyclophosphamide. Immunology. 1972 Oct;23(4):493–501. [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Voller A., O'Neill P. Immunofluorescence method suitable for large-scale application to malaria. Bull World Health Organ. 1971;45(4):524–529. [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]


