Abstract
The parasite burdens of livers and spleens of several strains of mice following infection with L. donovani were determined for a period of over 120 days. The parasite loads of the spleens and livers were correlated with the development of immunity to reinfection, footpad sensitivity and antibody titres. The strains of mice studied could be divided into two groups--those highly susceptible and those relatively non-susceptible. The antibody in the susceptible strains appeared 12 days after infection and increased thereafter. The antibody in the less susceptible strains appeared some time later and remained at the same level throughout the test period. In strains CAFl, C57B1/6 and BALB/c skin reactivity to Leishmania antigen developed at the time when the parasite burden had decreased from its peak. Skin reactivity never developed in DBA!l mice, the most highly susceptible strain, and in A/j the least susceptible strain. The development of resistance to reinfection with L. donovani following i.v. and i.p. infection was observed in C57Bl mice. Upon cell transfer from these immune animals, it was found that protective immunity was mediated through the thymus-dependent lymphocytes. Serum from immune mice was not able to confer immunity to the recipient animals.
Full text
PDF

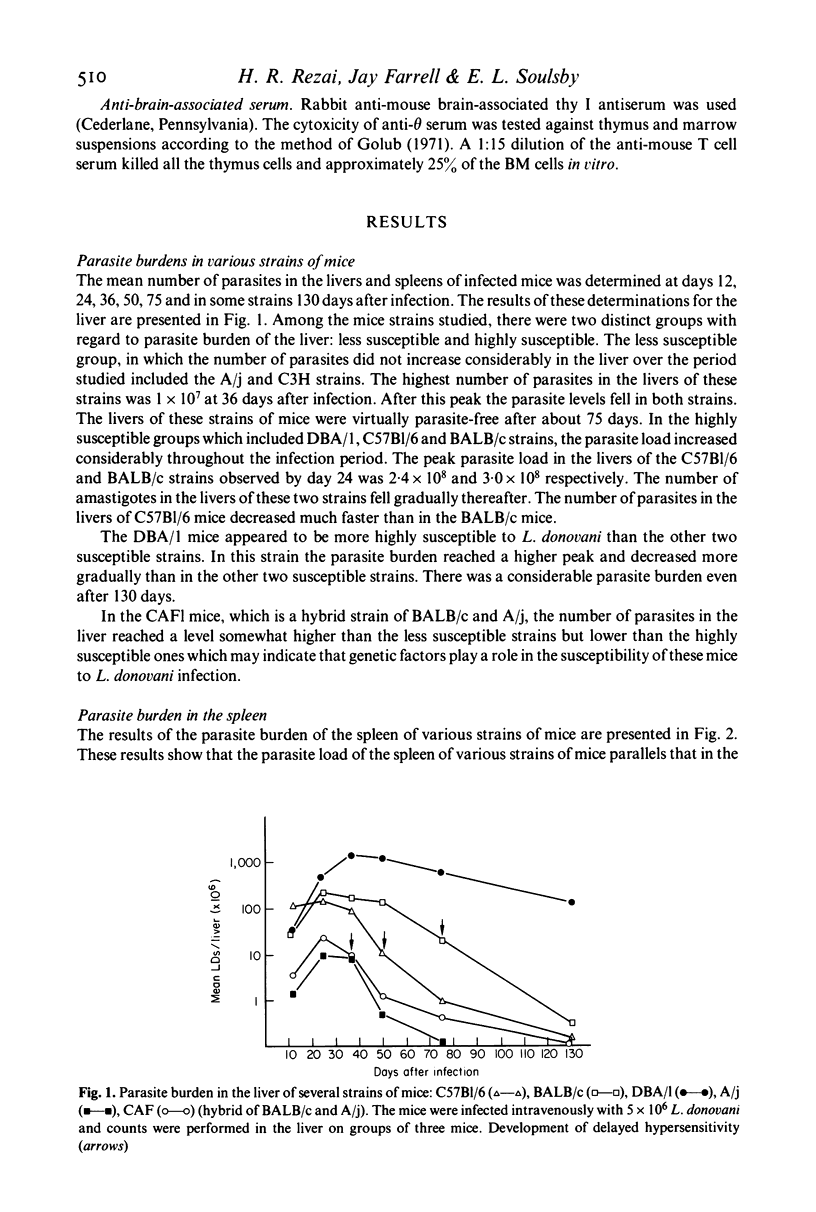


Selected References
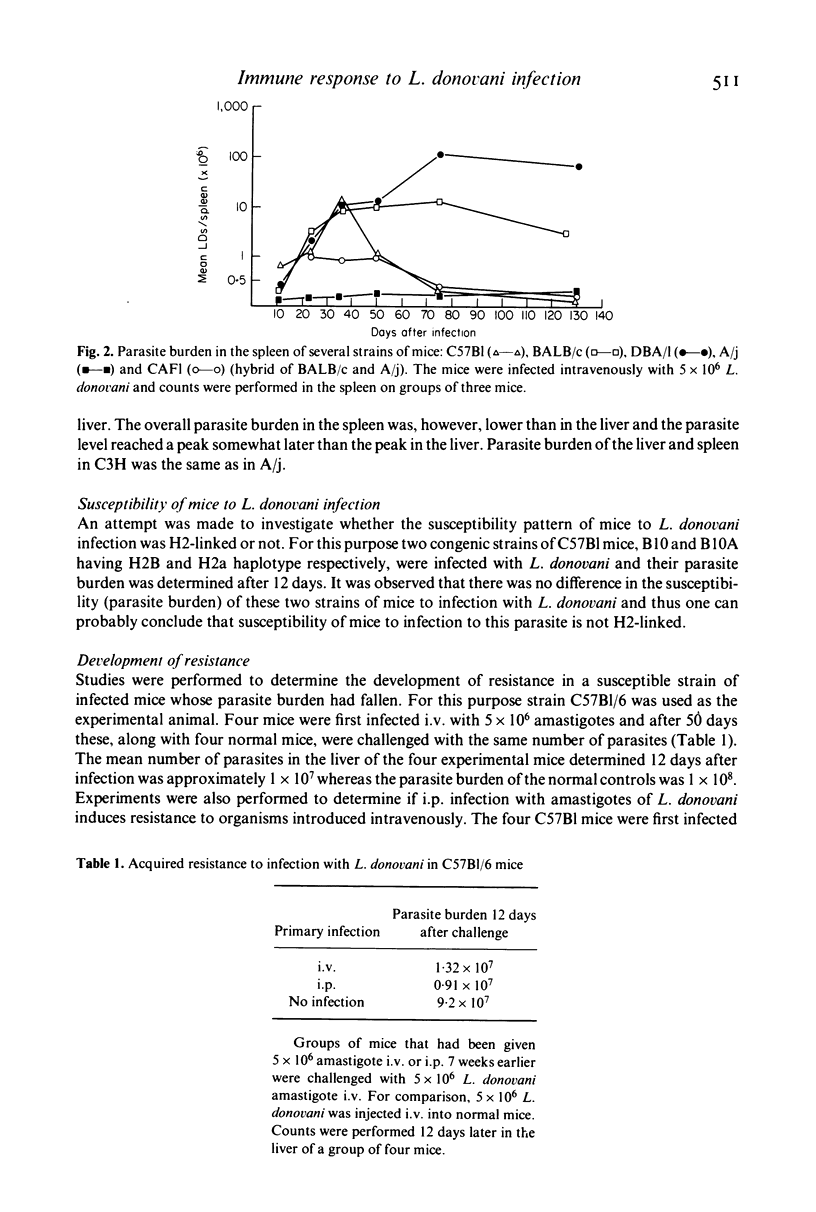
These references are in PubMed. This may not be the complete list of references from this article.
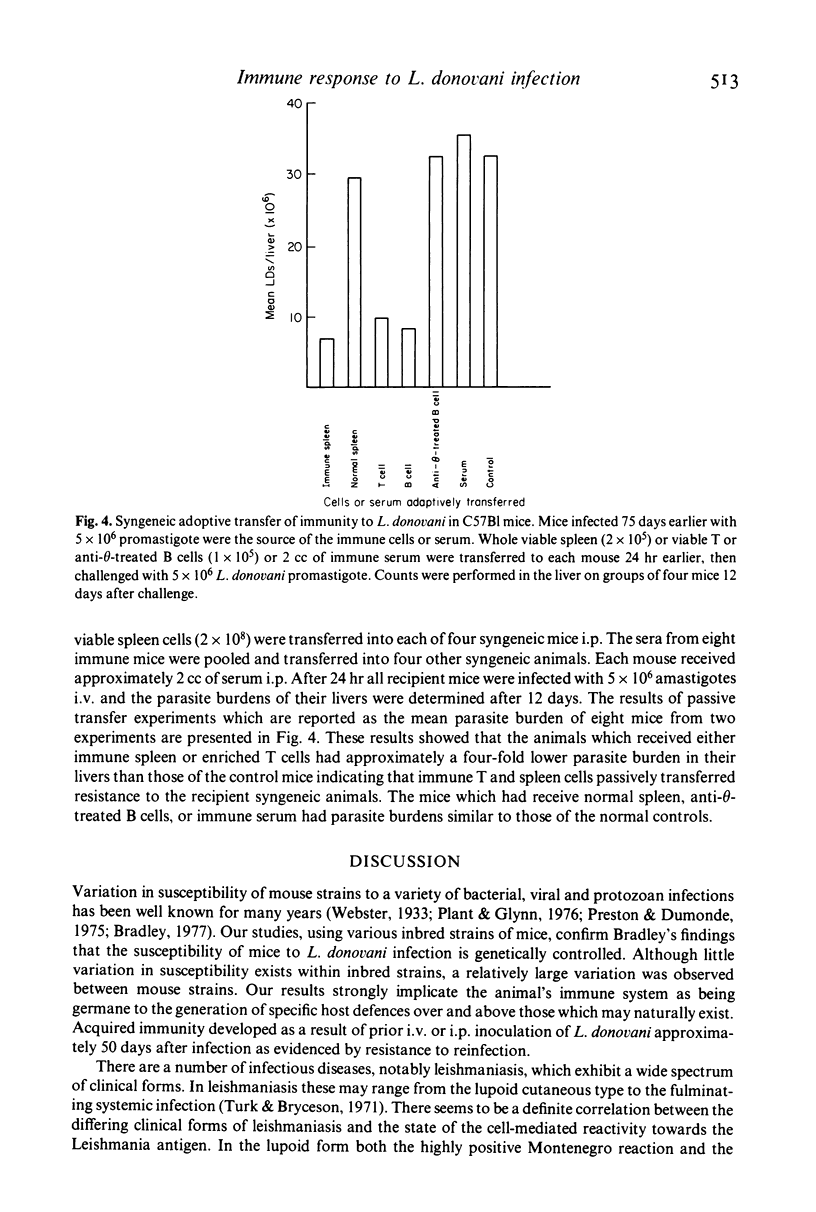
- Behforouz N., Rezai H. R., Gettner S. Application of immunofluorescence to detection of antibody in Leishmania infections. Ann Trop Med Parasitol. 1976 Sep;70(3):293–301. doi: 10.1080/00034983.1976.11687125. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Bray R. S. Leishmania. Annu Rev Microbiol. 1974;28(0):189–217. doi: 10.1146/annurev.mi.28.100174.001201. [DOI] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- MANSON-BAHR P. E. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961 Nov;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Ardehali S. M., Amirhakimi G., Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978 Nov;27(6):1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Jackson L., Paul W. E. T lymphocyte-enriched murine peritoneal exudate cells. I. A reliable assay for antigen-induced T lymphocyte proliferation. J Immunol. 1975 Nov;115(5):1330–1338. [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]


