Abstract
The long-term viability of a forest industry in the Amazon region of Brazil depends on the maintenance of adequate timber volume and growth in healthy forests. Using extensive high-resolution satellite analyses, we studied the forest damage caused by recent logging operations and the likelihood that logged forests would be cleared within 4 years after timber harvest. Across 2,030,637 km2 of the Brazilian Amazon from 1999 to 2004, at least 76% of all harvest practices resulted in high levels of canopy damage sufficient to leave forests susceptible to drought and fire. We found that 16 ± 1% of selectively logged areas were deforested within 1 year of logging, with a subsequent annual deforestation rate of 5.4% for 4 years after timber harvests. Nearly all logging occurred within 25 km of main roads, and within that area, the probability of deforestation for a logged forest was up to four times greater than for unlogged forests. In combination, our results show that logging in the Brazilian Amazon is dominated by highly damaging operations, often followed rapidly by deforestation decades before forests can recover sufficiently to produce timber for a second harvest. Under the management regimes in effect at the time of our study in the Brazilian Amazon, selective logging would not be sustained.
Keywords: Brazil, forest disturbance, remote sensing, selective logging, tropical forest
Satellite observations show that deforestation, defined here as clear-cutting of forests for pasture, agricultural, urban, and other uses, is a major force of ecological change throughout tropical regions (1). The pattern and process of deforestation have thus received considerable attention in ecological, socioeconomic, and policy studies (2, 3). Other human-caused forest disturbances, such as from selective timber harvesting and fire, are also common in tropical forests (4–7).
Selective logging is an economically important land use that results in less forest damage than deforestation, but the amount of canopy damage associated with logging can leave tropical forests highly susceptible to drought and fire (5, 8–11). If canopy damage levels are low, then selective logging has relatively small immediate and long-term impacts on forest resources (12, 13). If damage levels are high, however, then fundamental ecological processes (e.g., regeneration and succession) can be radically altered (14–18). Moreover, deforestation has long been associated with logging according to the theory of “invasive forest mobility” (19), whereby logging roads permit settler access into forests (3). However, neither the amount of forest disturbance caused by selective logging nor the amount of logged forest converted to cleared land has been directly quantified over large regions of tropical forest.
Selective logging is a diffuse but ubiquitous forest disturbance that occurs throughout the Brazilian Amazon. Using the Carnegie Landsat Analysis System (CLAS; see Supporting Text, which is published as supporting information on the PNAS web site), we have shown that selective logging rates of 12,075–19,823 km2·yr−1 nearly matched or exceeded rates of deforestation in the Brazilian Amazon from 1999 to 2002 (6). Here we present a large-scale high-resolution analysis of logging intensity and logging–deforestation interactions in a region containing most of the land-use activity found in the Brazilian Amazon. Using CLAS satellite data products on selective logging extent, combined with new subpixel satellite observations of forest canopy gap fraction, we quantified the spatial distribution of forest damage levels throughout >46,000 km2 of logged forest distributed across 2,030,637 km2 of the Brazilian Amazon, and we tracked the rates of canopy closure after timber harvest. Canopy gap fraction is used here, because it determines the immediate and long-term impacts of logging by regulation of key processes such as photosynthetic rates and primary production; canopy energy and water balance; mammal and insect behavior; population dynamics; and, critically, the probability of fire (9, 20–23). We then combine the logging extent maps with satellite maps of deforestation provided by the Brazilian National Institute for Space Research [Instituto Nacional de Pesquisas Espaciais (INPE) 2005; www.obt.inpe.br] to quantify logging–deforestation transitions from 1999 to 2004.
Results and Discussion
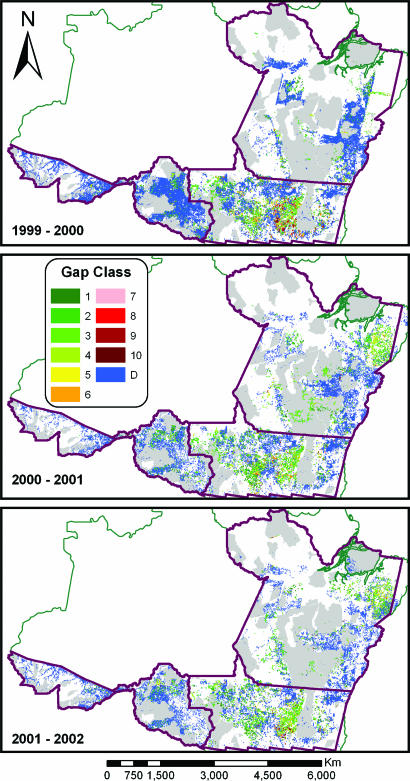
Satellite-based forest canopy gap fraction maps show the impact of timber harvest in area-integrated gap fractions (AIGF) over 1-km2 logging areas throughout the Amazon study region (Fig. 1). Between 1999 and 2002, 61–68% of all logging operations had AIGF values in the 10–40% range (Table 1). Gap fractions >10% represent high levels of damage that will leave the forest susceptible to fire under dry climatic conditions (10, 24–26). An additional 8–17% of all observed logging sites had extremely high levels of damage, with AIGF values exceeding 40%. The highest-damage levels were found mainly in northern Mato Grosso, but other high-damage timber harvests were noted throughout the region (Fig. 1). Although the highest-damage classes with AIGF values >80% may be construed as deforestation, they represented only 0.2–0.6% of all logging observed each year and were not reported in the INPE deforestation maps. Remarkably, we found that 21–24% of all logging observations were low damage (<10% AIGF); these levels are associated either with reduced-impact logging operations or very low (≪15 Mg·hectare−1) harvest intensities (24, 25, 27, 28). Overall, our results indicate that selective logging operations in the Brazilian Amazon were dominated by high-damage operations.
Fig. 1.
Annual logging intensity throughout a four-state region of the Brazilian Amazon. Logging intensity is expressed as AIGF at 1-km resolution. Gap classes range from 1 (0–10% AIGF) to 10 (>90% AIGF). Deforested areas (D; blue) are compiled from INPE (www.obt.inpe.br), with cumulative deforestation from 1997 to 2000 shown (Top) and annual increments for the subsequent years shown (Bottom). Gray areas show federal conservation lands and indigenous reserves.
Table 1.
Distribution of forest canopy gap classes for 1-km2 integrated areas in 46,043 km2 of logged sites spanning the years 2000-2002
| Gap class | Amazon, km2 |
||
|---|---|---|---|
| 2000 | 2001 | 2002 | |
| 1 | 4,246 | 3,386 | 2,918 |
| 2 | 6,629 | 5,827 | 4,771 |
| 3 | 3,436 | 2,667 | 2,233 |
| 4 | 2,072 | 1,178 | 1,094 |
| 5 | 1,470 | 574 | 509 |
| 6 | 979 | 317 | 279 |
| 7 | 577 | 152 | 136 |
| 8 | 263 | 65 | 71 |
| 9 | 102 | 29 | 35 |
| 10 | 16 | 3 | 8 |
| Total | 19,791 | 14,197 | 12,055 |
Gap classes range from 1 (0–10% AIGF) to 10 (≥90%). Methods for calculating AIGF are provided in Supporting Text.
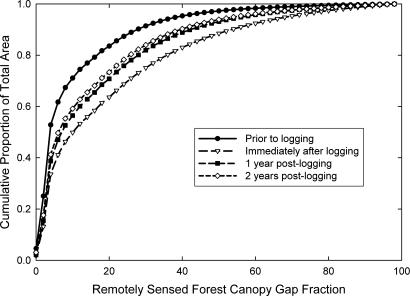
Distributions of forest canopy gap fraction yield an understanding of the regional variability of canopy opening before and after logging events. Changes in these distributions allow us to track the recovery of photosynthetic capacity through time to canopy closure. We caution that canopy closure rates do not correlate with the recovery of carbon stocks, because woody biomass regenerates far more slowly than leaf biomass (29, 30). We tracked forested areas before timber harvest (1999), immediately after logging (2000), and in subsequent years (2001–2002). Before logging, the distribution of canopy gaps was narrow, with more than half of the forest canopy having gap fractions <4% (Fig. 2). Selective logging increased the number of larger canopy gaps and left more than half of the forest with gap fractions in the 10–100% range. The distribution of gaps immediately after and for 2 years after logging indicated a significantly more open canopy than in the same forests before harvesting (Kolmogorov–Smirnov test; P < 0.02; Supporting Text). The distribution of canopy gaps 1 year after harvest indicated a recovery of forest leaf cover, but the rate of closure decreased and became insignificant after the first year of recovery (Kolmogorov–Smirnov test; P = 0.18; Fig. 2).
Fig. 2.
Cumulative forest gap fraction (30-m scale) distributions for selectively logged areas before harvest in 1999, after initial harvest in 2000, and 1 and 2 years after logging in 2001 and 2002, respectively.
Larger clearings caused by logging have faster proportional rates of recovery than smaller clearings, but they require substantially more time to reestablish their full canopy cover (see Supporting Text). Therefore, a disproportionate increase in larger gaps can be expected to slow overall forest recovery rates and thus will have long-lasting effects on a wide range of ecological processes from carbon cycling to faunal dynamics (16, 30, 31). These results also highlight the value of reduced-impact logging operations that tend to minimize initial canopy damage levels and thus are more conducive to an expeditious reestablishment of ecosystem functions (15, 27, 32). Low-impact or low-volume logging also leaves the forest with fewer and smaller canopy gaps, which significantly reduces the risk of fire (26).
Our combined analysis of logging and deforestation showed that each contributed an average of 15,383 km2·yr−1 and 17,200 km2·yr−1, respectively, or 32,583 km2·yr−1 in total, to the regional mosaic of forest change in four Brazilian states (Fig. 1). Both logging and deforestation were concentrated within 25 km of major roads. Federal conservation lands and indigenous reserves both suffered far fewer impacts of logging and deforestation than unprotected lands (Fig. 1). However, logged areas had a high probability of deforestation. Between 1999 and 2004, ≈16% of selectively logged forests were subsequently deforested within 1 year (Table 2). Deforestation of previously logged forest then increased 5.4% each year after initial timber harvest (r2 = 0.99, P < 0.001), resulting in a mean conversion rate of 32.7% in 4 years.
Table 2.
Percentage of logged forest subsequently deforested at 1–4 years after initial disturbance
| State | Logging year | Percent deforestation in year |
|||
|---|---|---|---|---|---|
| 2001 | 2002 | 2003 | 2004 | ||
| Acre | 2000 | 10.3 | 16.1 | 19.2 | 20.7 |
| Acre | 2001 | 38.7 | 47.3 | 50.5 | |
| Acre | 2002 | 38.9 | 43.1 | ||
| Rondônia | 2000 | 14.3 | 20.0 | 27.3 | 33.5 |
| Rondônia | 2001 | 16.2 | 22.9 | 28.2 | |
| Rondônia | 2002 | 27.3 | 33.5 | ||
| Mato Grosso | 2000 | 16.9 | 22.1 | 27.3 | 32.1 |
| Mato Grosso | 2001 | 12.4 | 17.4 | 22.2 | |
| Mato Grosso | 2002 | 14.5 | 19.5 | ||
| Pará | 2000 | 14.2 | 31.5 | 33.5 | 35.8 |
| Pará | 2001 | 20.9 | 22.8 | 25.4 | |
| Pará | 2002 | 20.6 | 23.5 | ||
| Total | 2000 | 16.3 | 23.5 | 28.2 | 32.7 |
| Total | 2001 | 15.1 | 19.4 | 23.6 | |
| Total | 2002 | 17.1 | 21.6 | ||
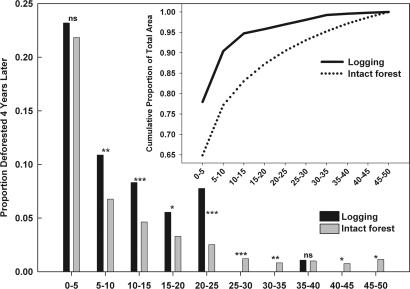
The probability of logged areas becoming deforested depends highly upon distance from major roads (Fig. 3). In the 5-km belt immediately adjacent to roads that contain nearly 80% of all logging activities, the probability of deforestation after logging did not differ significantly from the probability of clearing of intact forest. However, we found that the probability of clearing previously logged forest was two to four times greater than that of intact forest at distances of 5–25 km from main roads (Fig. 3). At these intermediate distances, logging clearly precedes deforestation. At distances much further from roads (>25 km), logging is almost nonexistent, and deforestation dominates the total forest disturbance regime.
Fig. 3.
The proportion of year 2000 logging and intact forest subsequently deforested by 2004 across the Amazon study area at distance classes of 0–50 km from major roads. The Pearson significance of χ2 comparisons between the deforestation of logged or intact points per distance class is shown as: ns, nonsignificant; ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001. (Inset) The cumulative distribution of logged forest (1999–2002) and intact forest as a function of distance from main roads.
Conclusions
The spatial coherence of logging and deforestation was expected, because both processes depend on road access. However, the high probability of clearing after logging (32% within 4 years) was unexpected, because logging and deforestation, each conducted by different agents (loggers vs. ranchers and farmers), are treated separately both by studies of land use change (33) and in the context of Brazilian environmental regulation. Our analysis suggests that, during the early years of this decade, forests were logged simply to convert ecological capital into economic capital to support other investments on the frontier (34). Furthermore, the predominance of destructive harvest methods left the forests susceptible to drought and fires for years after logging, threatening the long-term health and economic productivity of the forests. Recently, the Brazilian government approved new legislation to regulate forests lands and the timber industry. This new legislation may direct logging away from private land onto public concessions maintained for forest management. This new policy has the intention and potential to maintain forests under long-term timber management.
Materials and Methods
We used Landsat 7 Enhanced Thematic Mapper-Plus (ETM+) satellite data to quantify logging extent and intensity in the Brazilian states of Acre, Pará, Rondônia, and Mato Grosso (northern 58% of the state containing most of the forested area). These four states contain nearly all of the deforestation reported in the Brazilian Legal Amazon (INPE 2005; www.obt.inpe.br). This strategy required 120 images per year or 480 images in total. The logging-extent maps were derived at a spatial resolution of 30 × 30 m by using CLAS (6) (Figs. 4 and 5 and Tables 3 and 4, which are published as supporting information on the PNAS web site).
The CLAS algorithm provided estimates of canopy damage as expressed in the fraction of green photosynthetic vegetation (PV) cover within each Landsat image pixel. PV fractional cover is a radiometric quantity, and thus it cannot be directly measured in the field. In a series of field studies in the Brazilian Amazon, we developed equations relating subpixel PV fraction to canopy hemispherical gap fraction (6, 24, 25). Canopy hemispherical gap fraction (hereafter, gap fraction) is a key measure of canopy structure and canopy opening in forest ecosystems (35, 36). After selective logging, gap fraction is well correlated with ground damage, harvest intensity expressed as timber volume removed, and collateral vegetation damage (28, 31). We converted the PV images to gap fraction estimates at 30-m resolution across 2.3 million km2 of Amazon forest. To scale these high-resolution gap fraction images to the regional level, we calculated the AIGF defined as the mean of forest canopy gap fractions in 1-km2 cells (Fig. 6, which is published as supporting information on the PNAS web site). Although AIGF maps at 1-km2 resolution lose the details of canopy gap dynamics at the field scale, they provide a consistent metric to understand the regional patterns of forest canopy damage caused by logging and to monitor canopy closure over large areas in the years after selective harvest (Figs. 7 and 8 and Tables 5 and 6, which are published as supporting information on the PNAS web site).
The CLAS logging-extent maps were also combined with 30-m resolution deforestation maps from the INPE Digital Programa de Cálculo do Deflorestamento de Amazônia (www.obt.inpe.br) to analyze transitions from initial selective timber harvest to clear-cutting. Temporal lags between each logging event and subsequent forest clear-cutting events were analyzed in 24,378 polygons among four Brazilian states (Tables 3 and 4). Our analytical uncertainty was propagated from several component sources through to the final results, with the overall error from all sources combined averaged 13.45% (Tables 7 and 8, which are published as supporting information on the PNAS web site).
A land-use transition analysis was carried out to compare the probability of deforestation after logging or from intact forest. Using a digital map of main roads in the Brazilian Amazon, we created 5-km zones leading outward from each road to a maximum distance of 50 km (Fig. 9, which is published as supporting information on the PNAS web site), which is based on previous studies showing that the majority of deforestation occurs within a 50-km buffer around roads (37, 38). The analysis included a random selection of at least 1,000 logged and intact forest points per 5-km buffer. We then tracked the fate of those points for a period of 4 years (Fig. 10, which is published as supporting information on the PNAS web site). Details of this method are also provided in Supporting Text.
Supplementary Material
Acknowledgments
We thank M. Bustamante, B. Haxo, F. Merry, R. Raybin, and J. Zweede for assistance in the field and remote sensing portions of this study; C. Souza, Jr., for extremely helpful discussions; L. Curran and an anonymous reviewer for suggestions on the manuscript; the INPE Program for Monitoring Deforestation in the Brazillian Amazon for making its geographic information system coverages of deforestation freely available on the World Wide Web; and the Instituto do Homem e Meio Ambiente da Amazônia for the geographic information system coverage of major roads. CLAS was developed by the Carnegie Institution of Washington. The application of CLAS to Amazonia was funded by National Aeronautics and Space Administration Grant NNG-06-GC-88G for Large Scale Biosphere-Atmosphere Experiment in Amazonia-ECO Group LC-33.
Abbreviations
- CLAS
Carnegie Landsat Analysis System
- AIGF
area-integrated gap fraction(s)
- INPE
Instituto Nacional de Pesquisas Espaciais
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 12663.
References
- 1.Achard F., Eva H. D., Stibig H. J., Mayaux P., Gallego J., Richards T., Malingreau J. P. Science. 2002;297:999–1002. doi: 10.1126/science.1070656. [DOI] [PubMed] [Google Scholar]
- 2.Geist H. J., Lambin E. F. What Drives Tropical Deforestation? A Meta-Analysis of Proximate and Underlying Causes of Deforestation Based on Subnational Case Study Evidence (Land Use and Cover Change International Project Office, Louvain-la-Neuve, Belgium), 2001 LUCC Report Series no. 4. [Google Scholar]
- 3.Walker R. Ann. Assoc. Am. Geogr. 2003;93:376–398. [Google Scholar]
- 4.Nepstad D. C., Verissimo A., Alencar A., Nobre C., Lima E., Lefebvre P., Schlesinger P., Potter C., Moutinho P., Mendoza E., et al. Nature. 1999;398:505–508. [Google Scholar]
- 5.Cochrane M. A. Nature. 2003;421:913–919. doi: 10.1038/nature01437. [DOI] [PubMed] [Google Scholar]
- 6.Asner G. P., Knapp D. E., Broadbent E. N., Oliveira P. J. C., Keller M., Silva J. N. M. Science. 2005;310:480–482. doi: 10.1126/science.1118051. [DOI] [PubMed] [Google Scholar]
- 7.Lentini M., Pereira D., Celentano D., Pereira R. Fatos Florestais de Amazônia. Belém, Brazil: Instituto do Homem e Meio Ambiente da Amazônia; 2005. [Google Scholar]
- 8.Uhl C., Kauffman B. J. Ecology. 1990;71:437–449. [Google Scholar]
- 9.Holdsworth A. R., Uhl C. Ecol. Appl. 1997;7:713–725. [Google Scholar]
- 10.Nepstad D., Lefebvre P., Da Silva U. L., Tomasella J., Schlesinger P., Solorzano L., Moutinho P., Ray D., Benito J. G. Global Change Biol. 2004;10:704–717. [Google Scholar]
- 11.Van Nieuwstadt M. G. L., Sheil D. J. Ecol. 2005;93:191–201. [Google Scholar]
- 12.Sist P., Nguyen-The N. For. Ecol. Manage. 2002;165:85–103. [Google Scholar]
- 13.Bertault J. G., Sist P. For. Ecol. Manage. 1997;94:209–218. [Google Scholar]
- 14.Panfil S., Gullison R. For. Ecol. Manage. 1998;102:235–243. [Google Scholar]
- 15.Sist P., Nolan T., Bertault J.-G., Dykstra D. For. Ecol. Manage. 1998;108:251–260. [Google Scholar]
- 16.Fimbel R. A., Grajal A., Robinson J. G. The Cutting Edge: Conserving Wildlife in Logged Tropical Forests. New York: Columbia Univ. Press; 2001. [Google Scholar]
- 17.Putz F. E., Blate G. M., Redford K. H., Fimbel R., Robinson J. Conserv. Biol. 2001;15:7–20. [Google Scholar]
- 18.Gerwing J. J. For. Ecol. Manage. 2002;157:131–141. [Google Scholar]
- 19.Myers N. Environ. Conserv. 1980;7:101–114. [Google Scholar]
- 20.Bray J. R. Ecology. 1956;37:598–600. [Google Scholar]
- 21.Brokaw N. V. Ecology. 1985;66:682–687. [Google Scholar]
- 22.Mulkey S. S., Pearcy R. W. Funct. Ecol. 1992;6:719–729. [Google Scholar]
- 23.Murray K. G. Ecol. Monogr. 1988;58:271–298. [Google Scholar]
- 24.Asner G. P., Keller M., Silva J. N. M. Global Change Biol. 2004;10:765–783. [Google Scholar]
- 25.Asner G. P., Keller M., Pereira R., Zweede J. C., Silva J. N. M. Ecol. Applic. 2004;14:S280–S298. [Google Scholar]
- 26.Ray D., Nepstad D., Moutinho P. Ecol. Applic. 2005;15:1664–1678. [Google Scholar]
- 27.Holmes T. P., Blate G. M., Zweede J. C., Pereira R., Jr., Barreto P., Boltz F., Bauch R. For. Ecol. Manage. 2001;5583:1–18. [Google Scholar]
- 28.Pereira R., Zweede J., Asner G. P., Keller M. For. Ecol. Manage. 2002;168:77–89. [Google Scholar]
- 29.Silva J. N. M., Decarvalho J. O. P., Lopes J. D. A., Dealmeida B. F., Costa D. H. M., Deoliveira L. C., Vanclay J. K., Skovsgaard J. P. For. Ecol. Manage. 1995;71:267–274. [Google Scholar]
- 30.Keller M., Asner G. P., Silva J. N. M., Palace M. In: Working Forests in the Neotropics: Conservation Through Sustainable Management? Zarin D. J., Alavalapati J. R. R., Putz F. E., Schmink M., editors. New York: Columbia Univ. Press; 2004. pp. 41–63. [Google Scholar]
- 31.Keller M., Palace M., Asner G. P., Pereira R., Silva J. N. M. Global Change Biol. 2004;10:784–795. [Google Scholar]
- 32.Olander L. P., Bustamante M. M., Asner G. P., Telles E., Prado Z., Camargo P. B. Earth Interact. 2005;9:1–19. [Google Scholar]
- 33.Lambin E. F., Geist H. J., Lepers E. Annu. Rev. Environ. Res. 2003;28:205–241. [Google Scholar]
- 34.Vincent J. R., Binkley C. S. In: Managing the World's Forests, Sharma N. P., editor. Dubuque, IA: Kendall/Hunt; 1992. pp. 93–139. [Google Scholar]
- 35.Watt A. S. J. Ecol. 1947;35:1–22. [Google Scholar]
- 36.Denslow J. S. Annu. Rev. Ecol. Syst. 1987;18:431–451. [Google Scholar]
- 37.Nepstad D., Carvalho G., Barros A. C., Alencar A., Capobianco J. P., Bishop J., Moutinho P., Lefebvre P., Silva U. L., Jr., Prins E. For. Ecol. Manage. 2001;154:395–407. [Google Scholar]
- 38.Alves D. S. Int. J. Remote Sens. 2002;23:2903–2908. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.