Abstract
Double-stranded RNA (dsRNA) is a by-product of viral RNA polymerase activity, and its recognition is one mechanism by which the innate immune system is activated. Cellular responses to dsRNA include induction of alpha/beta interferon (IFN) synthesis and activation of the enzyme PKR, which exerts its antiviral effect by phosphorylating the eukaryotic initiation factor eIF-2 alpha, thereby inhibiting translation. We have recently identified the nonstructural protein NSs of Bunyamwera virus (BUNV), the prototype of the family Bunyaviridae, as a virulence factor that blocks the induction of IFN by dsRNA. Here, we investigated the potential of NSs to inhibit PKR. We show that wild-type (wt) BUNV that expresses NSs triggered PKR-dependent phosphorylation of eIF-2 alpha to levels similar to those of a recombinant virus that does not express NSs (BUNdelNSs virus). Furthermore, the sensitivity of viruses in cell culture to IFN was independent of PKR and was not determined by NSs. PKR knockout mice, however, succumbed to infection approximately 1 day earlier than wt mice or mice deficient in expression of RNase L, another dsRNA-activated antiviral enzyme. Our data indicate that (i) bunyaviruses activate PKR, but are only marginally sensitive to its antiviral effect, and (ii) NSs is different from other IFN antagonists, since it inhibits dsRNA-dependent IFN induction but has no effect on the dsRNA-activated PKR and RNase L systems.
The family Bunyaviridae is a large group of viruses which contains more than 300 named virus isolates (7). They are mainly transmitted by arthropods, and several members cause encephalitis, febrile illnesses, or hemorrhagic fevers, e.g., La Crosse, Oropouche, Hantaan, Rift Valley fever, and Crimean-Congo hemorrhagic fever viruses, in humans (8). The family has been classified into five genera: Bunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus. The prototype of both the family Bunyaviridae and the genus Bunyavirus is Bunyamwera virus (BUNV), and this is the only family member to date that has been rescued from cloned cDNAs (3). All bunyaviruses are enveloped and have a trisegmented, single-stranded RNA genome of negative polarity, replicate in the cytoplasm, and bud into the Golgi apparatus (7). They encode four common structural proteins: the viral polymerase (L) on the large (L) segment, two glycoproteins (G1 and G2) on the medium (M) segment, and the viral nucleocapsid protein (N) on the smallest (S) segment. Viruses within some genera also encode nonstructural proteins, either on the M segment (termed NSm) or on the S segment (NSs).
Alpha/beta interferons (IFN-α and -β) are a major component of the innate immune system. As a reaction to virus infection, cells synthesize and secrete IFNs that initiate synthesis of antiviral proteins in the target cell, thus establishing an antiviral state (20). Induction of IFN occurs at the level of transcriptional initiation and requires several regulatory factors that can be activated by different triggers, the most important being double-stranded RNA (dsRNA), a by-product of viral RNA polymerases. To combat different types of viruses, multiple, partially redundant pathways have evolved that inhibit different stages of virus replication. The best characterized antiviral factors induced by IFN-α and -β are the Mx proteins (12), the 2′-5′ oligoadenylate synthetase (2-5 OAS)/RNase L system (19), and the protein kinase R (PKR) (24). Mx proteins are large GTPases that inhibit the multiplication of several RNA viruses, including representative members of the Bunyavirus, Hantavirus, and Phlebovirus genera within the Bunyaviridae family (9, 13-15, 18). For La Crosse virus, which is closely related to BUNV, it has been shown that the Mx protein sequesters the N protein, thereby depleting the viral RNA synthesis machinery from a factor that is essential for replicating the genome (16). By contrast, little is known about the activity of 2-5 OAS/RNase L and PKR against bunyaviruses. Both systems are constitutively expressed in the cell, upregulated by IFN, and become activated following interaction with dsRNA. The 2-5 OAS catalyzes the synthesis of short 2′-5′ oligoadenylates that activate the latent endoribonuclease RNase L. RNase L, in turn, then degrades both viral and cellular RNAs, thus inhibiting viral replication (27). PKR is a serine-threonine kinase that phosphorylates the α subunit of eukaryotic translation initiation factor eIF2, thereby sequestering the recycling factor, eIF2B/GEF (20). As a consequence, translation of cellular and viral mRNAs is inhibited. Thus, the effector proteins activated by IFN protect the host by blocking replication, transcription, or translation of viral genes.
Viruses have evolved different compensatory defense mechanisms to block either IFN induction, IFN signal transduction, or the action of particular antiviral proteins (10, 11). Prominent examples of such IFN antagonists are the NS1 protein of influenza A virus (FLUAV) (10) and the E3L protein of poxviruses (5, 25), which are dsRNA binding proteins. Thus, NS1 and E3L not only interfere with synthesis of IFN but also increase resistance to IFN by inhibiting activation of the PKR/2-5 OAS systems. It was reported recently that the NSs protein of BUNV inhibits the production of IFN in infected cells (23). A recombinant BUNV was generated that does not express the NSs protein (4), and it was demonstrated that this virus, named BUNdelNSs, but not the wild-type (wt) BUNV counterpart, is a strong inducer of IFN-α and -β. Consequently, BUNdelNSs virus replicated to levels similar to those of wt BUNV in both cells and mice lacking a functional IFN-α and -β system but was restricted in IFN-competent systems. IFN induction by BUNdelNSs virus was found to involve virally produced dsRNA and the dsRNA-activated transcription factors NF-κB and IRF-3. Furthermore, transiently expressed NSs was found to be sufficient to inhibit activation of an IFN promoter by dsRNA, indicating that NSs can act independently of viral replication, somehow interfering with the signal transduction pathway that is activated by dsRNA.
Here, we investigated whether BUNV NSs behaves in a manner similar to that of other IFN antagonists and—besides inhibiting the dsRNA-dependent IFN induction—inhibits also the activation of the dsRNA-dependent PKR enzyme. Furthermore, the general sensitivity of BUNV against IFN, and in particular against the dsRNA-dependent effectors PKR and 2-5 OAS, was evaluated.
Activation of PKR.
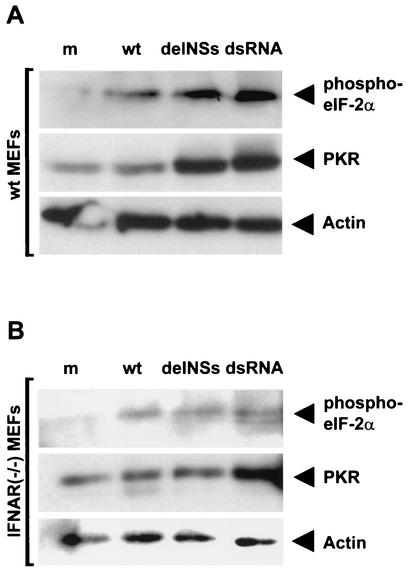
To investigate whether BUNV NSs would influence PKR, we assayed phosphorylation of the eukaryotic translation factor eIF-2α in infected cells. Murine embryonic fibroblasts (MEFs) derived from wt 129 mice (kindly donated by Jovan Pavlovic, University of Zurich, Switzerland) were either mock infected or infected with wt BUNV or BUNdelNSs virus or transfected with dsRNA as a positive control. The phosphorylation of eIF-2α was detected by immunoblot analysis of the cell lysates. Figure 1A (upper row) shows that wt BUNV, BUNdelNSs virus, and transfected dsRNA induce eIF-2α phosphorylation, whereas in untreated cells, no signal was detected. wt BUNV induced eIF-2α phosphorylation to a lower level than dsRNA or BUNdelNSs virus. PKR expression, however, was elevated in BUNdelNSs-infected and in dsRNA-treated cells, whereas wt BUNV-infected cells contained amounts of PKR similar to that of the mock-treated control (Fig. 1A, middle row). The actin control demonstrates that comparable amounts of protein were used in all lanes (Fig. 1A, lower row). As an additional control, we verified that neither wt BUNV, BUNdelNSs virus, nor dsRNA was able to induce a change in eIF-2α phosphorylation in cells derived from PKR knockout mice (data not shown). Thus, induction of eIF-2α phosphorylation by wt BUNV and BUNdelNSs virus requires dsRNA-dependent PKR and is a reliable marker of PKR activity in this system.
FIG. 1.
Activation of PKR in infected wt (A) or IFNAR(−/−) (B) cells. Cells were either mock infected (lanes m), infected with 5 PFU/cell of wt BUNV (lanes wt) or BUNdelNSs virus (lanes delNSs), or transfected with 10 μg of poly(I-C) (lanes dsRNA). After 16 h, cells were lysed in buffer containing 50 mM Tris-HCl (pH 8), 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM dithiothreitol, 10 mM NaF, 0.25 mM NaVO4, and protease inhibitor cocktail mix (Roche). Lysates (10 μg of protein per lane) were assayed by Western blot analysis with antibodies against phosphorylated eIF-2α (1:200 dilution of rabbit anti-eIF-2α [pS51]; Biosource International), PKR (1:200 dilution of mouse monoclonal antibody B-10; Santa Cruz Biotechnology), or actin as a control (1:500 dilution of mouse monoclonal antibody AC-40; Sigma). Protein bands were visualized using the ECL method (Amersham).
Increased PKR expression and eIF-2α phosphorylation in BUNdelNSs-infected cells relative to wt BUNV-infected cells most probably resulted from IFN induced by the BUNdelNSs virus. To determine whether this was the case, we investigated eIF-2α phosphorylation in MEF cells unresponsive to IFN due to absence of the IFN-α/β receptor [IFNAR(−/−) MEFs] (17). In Fig. 1B (upper row), it is shown that eIF-2α phosphorylation was similar in IFNAR(−/−) MEFs infected with wt BUNV or BUNdelNSs virus or those treated with dsRNA, whereas in mock-treated cells, no such signal could be detected. Furthermore, PKR was expressed to similar levels (Fig. 1B, middle row), as was actin (Fig. 1B, lower row), suggesting that elevated PKR expression observed in wt cells was indeed due to IFN induced by BUNdelNSs virus or dsRNA and that this resulted in increased eIF-2α phosphorylation.
Taken together, these data suggest that BUNV produces dsRNA that activates PKR, which in turn leads to eIF-2α phosphorylation, and that the bunyavirus virulence factor NSs does not possess an activity to inhibit these events.
Effect of IFN and PKR on bunyavirus growth in cell culture.
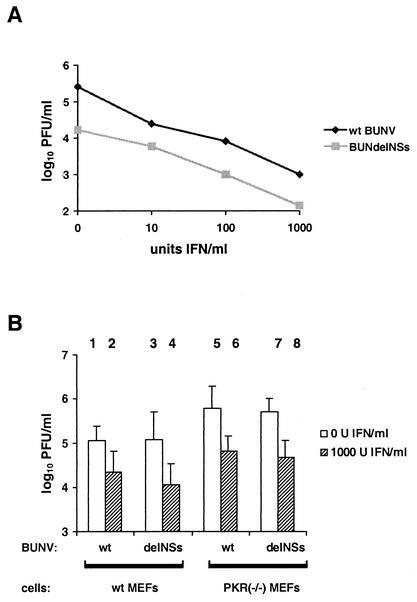
To determine whether NSs can contribute to resistance against the antiviral action of IFN, we compared the growth of wt BUNV and BUNdelNSs virus in IFN-treated cells. For these assays, we chose Vero cells because they are unable to synthesize IFN but are fully responsive to IFN treatment (6), thus excluding any additional effects of virus-induced IFN that would bias the results. Cells were pretreated with increasing doses of IFN-α and -β (10 to 1,000 IU/ml) and infected with virus, and infectious virus in the supernatant was determined after overnight incubation. As expected (4), wt BUNV had an approximately 10-fold-higher titer than BUNdelNSs virus in cells not treated with IFN. When increasing amounts of IFN were added, significant reductions of virus yields were observed. If plotted on a double logarithmic scale (Fig. 2A), titers of both wt BUNV and BUNdelNSs virus decreased linearly with a slope of approximately −0.7; i.e., a 10-fold increase in IFN concentration resulted in an 0.7-fold decrease of log10 virus titer. Since both lines appear parallel to each other, we conclude that both viruses have a similar sensitivity to IFN. Thus, BUNV is sensitive to the antiviral effects of IFN in Vero cells, and the NSs protein does not confer IFN resistance.
FIG. 2.
Effect of IFN and PKR in cell culture. (A) Vero cells were grown to 80% confluence and incubated overnight with various concentrations of human interferon alpha A/D (BglII) (PBL Biomedical Laboratories). After 24 h of incubation, cells were infected with 0.01 PFU/cell of wt BUNV or BUNdelNSs virus and incubated in Dulbecco's modified Eagle medium-10% fetal calf serum, again containing IFN. Virus yields were measured 18 h postinfection in plaque assays, as described previously (22). (B) IFN sensitivity of viruses in wt cells (columns 1 to 4) or PKR(−/−) MEF cells (columns 5 to 8). Cells were treated either with 1,000 neutralizing units of sheep polyclonal anti-mouse IFN-α/β (PBL Biomedical Laboratories) or with 1,000 units of IFN-α and -β for 24 h, infected with 0.01 PFU/cell of wt BUNV (columns 1, 2, 5, and 6) or BUNdelNSs virus (columns 3, 4, 7, and 8), and incubated in Dulbecco's modified Eagle medium-10% fetal calf serum, again containing IFN. Virus yields were measured 18 h postinfection with MEFs derived from IFN-α/β receptor knockout mice. Averages and standard deviations of results of three independent experiments are shown.
Next we investigated the anti-bunyavirus activity of PKR. To this aim, we measured the IFN effect in wt cells and MEF cells derived from PKR knockout mice [PKR(−/−) MEFs], kindly donated by Jovan Pavlovic, University of Zurich, Switzerland. Cells were incubated with IFN prior to infection, and virus growth was compared to that in untreated cells. A neutralizing anti-IFN antiserum was added to the supernatants of the untreated controls to exclude the effect of virus-induced IFN which would inhibit growth of BUNdelNSs (23). Furthermore, to exclude potential carryover effects of the IFN present in the supernatants of treated cells, we used IFN-insensitive IFNAR(−/−) MEFs for titrations. In Fig. 2B (columns 1 to 4), it is shown that in PKR-expressing wt cells, both wt BUNV and BUNdelNSs virus were inhibited to similar extents by IFN. Surprisingly, IFN also displayed an inhibitory effect against both viruses on PKR(−/−) cells that was not essentially different from that on wt cells (Fig. 2B, columns 5 to 8). Furthermore, only slightly higher titers were observed in the mutant cells relative to those in wt cells, despite the absence of PKR. We therefore investigated growth of the PKR-sensitive vesicular stomatitis virus (VSV) in our MEF cells. Similar to the situation with the bunyaviruses, no apparent contribution of PKR to the IFN effect was detected (data not shown). This suggests that the levels of activated PKR in our MEF cells are not sufficient to inhibit virus growth significantly. Taken together, these data indicate that, in MEF cells, BUNV is not sensitive to PKR action and that this mechanism is independent from the NSs virulence factor.
Effect of PKR and 2-5 OAS systems in vivo.
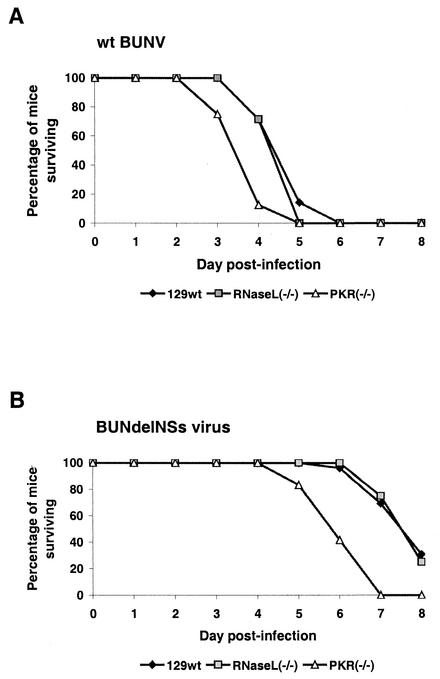
We investigated whether PKR has an in vivo effect against bunyaviruses by comparing survival of infected wt 129 mice and their PKR-deficient counterparts (26). Furthermore, we included mice lacking the 2-5 OAS system (27) to address the role of this dsRNA-dependent enzyme with respect to bunyavirus pathogenesis. Groups of mice expressing PKR (PKR 129 wt mice) or mice lacking PKR [PKR(−/−) mice] or the 2-5 OAS system [RNase L(−/−) mice] were inoculated intracerebrally with wt BUNV and observed for survival. Figure 3A shows that wt mice were killed within 5 days, as expected (4, 23). By contrast, mice lacking PKR succumbed to disease approximately 1 day earlier, suggesting that PKR has a certain protective effect against wt BUNV in vivo. Surprisingly, however, mice deficient in RNase L expression displayed no difference from wt mice with respect to disease progression, indicating that the 2-5 OAS system has no anti-bunyaviral activity. To determine whether the BUNV NSs virulence factor had any influence on the action of either PKR or 2-5 OAS in vivo, we infected wt and knockout mice in parallel with the BUNdelNSs mutant virus. As expected, the BUNdelNSs virus displayed a difference in onset of disease compared to wt BUNV (Fig. 3B), presumably due to the IFN induced by the NSs-deficient mutant (23). With respect to the sensitivity to PKR and 2-5 OAS, however, the situation was similar to that for wt BUNV, since PKR(−/−) mice succumbed to disease between 1 and 2 days earlier than wt mice, whereas for the RNase L-deficient mice, again there was no difference (Fig. 3B). To further investigate how PKR may affect virus growth in vivo, we determined the virus titers in brains of wt and PKR(−/−) animals (Table 1). As observed in cell culture (see Fig. 2B), wt BUNV grew to similar titers in wt mice and PKR(−/−) mice at day 3 postinfection. By contrast, growth of BUNdelNSs virus was inhibited by approximately 2 log steps in wt mice, but not in PKR(−/−) animals. This difference is most probably due to the IFN induced by the delNSs virus, which leads to increased expression of PKR in wt animals (see Fig. 1A), again suggesting that PKR has a certain anti-bunyaviral effect in vivo.
FIG. 3.
Virulence of bunyaviruses in 129 wt, PKR(−/−), and RNase L(−/−) mice. Groups of six 5-week-old specific-pathogen-free female mice (kindly obtained from Jovan Pavlovic, University of Zurich, Switzerland) were inoculated intracerebrally with 1,000 PFU of wt BUN (A) or BUNdelNSs (B) viruses. The animals were monitored twice daily over an 8-day period. Mice that were moribund or severely paralyzed were killed and scored dead on the day these symptoms were observed.
TABLE 1.
Virus growth in brains of wt mice and PKR (−/−) mice
| Virus | Mice | Titers (PFU/ml)a |
|---|---|---|
| wt | wt | 1.5 × 106 |
| PKR(−/−) | 1.7 × 106 | |
| BUNdelNSs | wt | 6 × 104 |
| PKR(−/−) | 1.4 × 106 |
Mice were sacrificed at day 3 postinfection.
Taken together, these results indicate that of the antiviral systems that are activated by dsRNA, PKR, but not 2-5 OAS, has a weak protective effect against BUNV in vivo. Furthermore, as demonstrated in cell culture, the NSs virulence factor does not contribute to viral resistance against any of these IFN effectors.
In this study, multiple aspects of bunyavirus interaction with the IFN system were investigated. Firstly, we demonstrated that the BUNV NSs protein, which was previously identified as a factor counteracting IFN induction (23), does not appear to interfere with activation of the antiviral enzyme PKR. Secondly, NSs does not influence the IFN sensitivity of the virus. Thirdly, although both wt BUNV and the NSs-deficient BUNdelNSs mutant activate PKR, a contribution of PKR to host resistance could be observed only in vivo. Lastly, the 2-5 OAS has no antiviral effect in vivo against either wt BUNV or BUNdelNSs virus.
Many viruses, e.g., influenza viruses and poxviruses, have evolved mechanisms to counteract both IFN induction and IFN action by sequestering of dsRNA (reviewed in references 10 and 11). In previous studies, the BUNV NSs protein appeared to act by a similar mechanism, since (i) infection with the BUNdelNSs mutant, but not with the NSs-expressing wt virus, led to a dsRNA-dependent induction of IFN, and (ii) NSs expressed in cell culture was able to inhibit dsRNA-mediated activation of an IFN promoter (23). Here, we clarified whether NSs also interferes with the dsRNA-activated IFN effectors PKR and 2-5 OAS. Surprisingly, NSs was unable to inhibit PKR activation (Fig. 1). Neither did NSs enhance virus resistance to IFN, since both wt BUNV and BUNdelNSs virus displayed similar dose-response curves to IFN (Fig. 2A). Furthermore, the impact of PKR on virus growth in cell culture (Fig. 2B) and of PKR and 2-5 OAS on disease progression in experimental animals (Fig. 3) was independent of the presence of NSs. Thus, unlike other IFN antagonists such as E3L of vaccinia virus (25) and NS1 of FLUAV (10), BUNV NSs appears to function exclusively at the level of induction of IFN by virally produced dsRNA without affecting activation of the dsRNA-dependent enzymes PKR and 2-5 OAS. In passing, we have been unable to demonstrate specific binding of BUNV NSs to dsRNA-coated Sepharose beads (F. Weber, unpublished results).
An unexpected feature of BUNV multiplication is that PKR is activated (Fig. 1) but does not have an apparent impact on virus growth in cell culture (Fig. 2B). Some protective effect was observed in the animal model (Fig. 3), but here also PKR was not the factor determining whether mice succumbed to infection or not. In line with these results, the highly related La Crosse virus is resistant to PKR in vivo (H. P. Hefti and J. Pavlovic, personal communication). Thus, these bunyaviruses are different from FLUAV and VSV, the best characterized negative-stranded RNA viruses with respect to PKR inhibition. For both of these viruses, it has been shown that PKR significantly contributes to the antiviral effect of IFN in cell culture and in mice. For FLUAV, growth of an NS1-deficient mutant in IFN-competent cells could be rescued to some extent by inhibiting PKR, and the mutant virus was able to grow in PKR(−/−) mice but not in wt mice (2). Similarly, wt FLUAV was able to kill 100% of PKR(−/−) mice, whereas less than 40% of wt mice succumbed to infection (1). For VSV, IFN-mediated growth inhibition was more pronounced in cells expressing PKR than in the PKR(−/−) counterpart, and PKR(−/−) mice succumbed to infection, whereas PKR-expressing mice survived (1, 21). Thus, despite the similarities of FLUAV and VSV to BUNV with respect to the genetic material (negative-stranded RNA), the segmented genome (FLUAV), and the cytoplasmic location of RNA synthesis (VSV), bunyaviruses appear to be partially resistant to PKR action. In addition, BUNV appears to be even more insensitive to the 2-5 OAS system (Fig. 3) which is also activated by dsRNA. To our knowledge, no report exists to date to demonstrate the interaction of bunyaviruses with these parts of the IFN response.
In summary, our studies demonstrate that the bunyavirus NSs virulence factor is different from other IFN antagonists, since it inhibits dsRNA-dependent IFN induction but has no effect on dsRNA-activated PKR. In addition, we showed that bunyaviruses are not highly sensitive to the antiviral effect of PKR and 2-5 OAS. Characterization of the interaction of bunyaviruses with the IFN system may help to better understand virus-host cell interactions and to find means for controlling the spread of the hazardous members of the Bunyaviridae family.
Acknowledgments
We thank Jovan Pavlovic for communicating unpublished results and for kindly providing cells and mice that were essential for this study. Furthermore, we thank Otto Haller for helpful discussions and support; Alain Kohl, Georg Kochs, Martin Spiegel, and Peter Staeheli for critically reading the manuscript; and Angela McLees for excellent technical assistance.
Work in the authors' laboratories is supported by Deutsche Forschungsgemeinschaft grants We 2616/1-1 and 1-2 (to F.W.), Wellcome Trust grants 048387 and 046745 (to R.M.E.), and MRC grant 52315 (to J.K.F.).
REFERENCES
- 1.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridgen, A., and R. M. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl. Acad. Sci. USA 93:15400-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgen, A., F. Weber, J. K. Fazakerley, and R. M. Elliott. 2001. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA 98:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz, M. O., S. Ziemin, M. M. Le Beau, P. Pitha, S. D. Smith, R. R. Chilcote, and J. D. Rowley. 1988. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. USA 85:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott, R. M. 1996. The Bunyaviridae. Plenum Press, New York, N.Y.
- 8.Elliott, R. M. 1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3:572-577. [PMC free article] [PubMed] [Google Scholar]
- 9.Frese, M., G. Kochs, H. Feldmann, C. Hertkorn, and O. Haller. 1996. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein J. Virol. 70:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Sastre, A. 2001. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology 279:375-384. [DOI] [PubMed] [Google Scholar]
- 11.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signaling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 12.Haller, O., M. Frese, and G. Kochs. 1998. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Tech. 17:220-230. [DOI] [PubMed] [Google Scholar]
- 13.Hefti, H. P., M. Frese, H. Landis, C. Di Paolo, A. Aguzzi, O. Haller, and J. Pavlovic. 1999. Human MxA protein protects mice lacking a functional alpha/beta interferon system against La Crosse virus and other lethal viral infections J. Virol. 73:6984-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin, H. K., K. Yoshimatsu, A. Takada, M. Ogino, A. Asano, J. Arikawa, and T. Watanabe. 2001. Mouse Mx2 protein inhibits hantavirus but not influenza virus replication. Arch. Virol. 146:41-49. [DOI] [PubMed] [Google Scholar]
- 15.Kanerva, M., K. Melen, A. Vaheri, and I. Julkunen. 1996. Inhibition of puumala and tula hantaviruses in Vero cells by MxA protein. Virology 224:55-62. [DOI] [PubMed] [Google Scholar]
- 16.Kochs, G., C. H. Janzen, H. Hohenberg, and O. Haller. 2002. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl. Acad. Sci. USA 99:3153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 18.Sandrock, M., M. Frese, O. Haller, and G. Kochs. 2001. Interferon-induced rat mx proteins confer resistance to Rift Valley fever virus and other arthropod-borne viruses. J. Interferon Cytokine Res. 21:663-668. [DOI] [PubMed] [Google Scholar]
- 19.Silverman, R. H. 1994. Fascination with 2-5A-dependent RNase: a unique enzyme that functions in interferon action. J. Interferon Res. 14:101-104. [DOI] [PubMed] [Google Scholar]
- 20.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 21.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74:9580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watret, G. E., C. R. Pringle, and R. M. Elliott. 1985. Synthesis of bunyavirus-specific proteins in a continuous cell line (XTC-2) derived from Xenopus laevis. J. Gen. Virol. 66:473-482. [DOI] [PubMed] [Google Scholar]
- 23.Weber, F., A. Bridgen, J. K. Fazakerley, H. Streitenfeld, R. E. Randall, and R. M. Elliott. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon J. Virol. 76:7949-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams, B. R. 1999. PKR; a sentinel kinase for cellular stress. Oncogene 18:6112-6120. [DOI] [PubMed] [Google Scholar]
- 25.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus J. Virol. 76:5251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, A., J. Paranjape, T. L. Brown, H. Nie, S. Naik, B. Dong, A. Chang, B. Trapp, R. Fairchild, C. Colmenares, and R. H. Silverman. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]