Abstract
Monocyte dysfunction in filarial infection has been proposed as one mechanism underlying the diminished antigen-specific T-cell response seen in patent lymphatic filariasis. Cytokine/chemokine production and gene expression in monocytes from filaria-infected patients and uninfected healthy donors were assessed unstimulated and in response to stimulation with Staphylococcus aureus Cowan I bacteria plus gamma interferon both before and 8 months following treatment. Monocytes from filaria-infected individuals were studded with intracellular microfilarial antigens. Furthermore, monocytes from these individuals were less capable of producing interleukin-8 (IL-8), Exodus II, MIP-1α, MIP-1β, and IL-1α and preferentially expressed genes involved in apoptosis and adhesion compared with monocytes from uninfected donors. Eight months following treatment with a single dose of ivermectin-albendazole, some of these defects were reversed, with monocyte production of IL-8, IL-1α, MIP-1α, and IL-10 being comparable to that seen in the uninfected controls. In addition, a marked increase in mRNA expression of genes associated with protein metabolism, particularly heat shock proteins, was seen compared with pretreatment expression. These data suggest that the function and gene expression of monocytes in filaria-infected patients are altered but that this dysfunction is partially reversible following antifilarial treatment.
Lymphatic filariasis is caused most commonly by infection with the nematodes Wuchereria bancrofti and Brugia malayi and is associated with varied clinical outcomes. The most intriguing is a subclinical condition associated with the inability of T cells to proliferate or produce gamma interferon (IFN-γ) in response to parasite antigen (19), a finding that rarely extends to non-parasite- or mitogen-driven responses (reviewed in reference 20). The lack of T-cell responsiveness has been shown to be directed primarily at antigens produced by microfilariae (MF) found in the circulation (17), and a multitude of mechanisms, including regulatory cytokines (13), T-cell apoptosis (10), inducible nitric oxide synthase (7), pro- and anti-inflammatory cytokines, and altered function of antigen-presenting cells (APC) (16, 27, 31), have been postulated to underlie this defect.
The role of APC in induction or maintenance of the immune response in W. bancrofti infection has been examined in humans only using in vitro systems. We have previously shown that MF antigen impairs production of interleukin-12 (IL-12) and IL-10 by monocyte-derived dendritic cells (DC) (27) and that the infective stage of B. malayi (L3) results in suppression of the function of epidermal Langerhans' cells and thus leads to a decreased ability of Langerhans' cells to promote CD4+ T-cell activation in an allogeneic mixed lymphocyte reaction (25). Murine macrophages have been shown to be involved in the killing of filarial parasites, in the pathology associated with dying worms, and in mediating immune hyporesponsiveness (reviewed in reference 2). Moreover, compared with responses seen in human macrophages exposed to or infected with intracellular pathogens (5), filarial L3 was much less capable of inducing gene expression than were other parasitic pathogens.
Monocytes in filarial infection have been described, in part, as a major source of IL-10 production in filaria-infected patients (18). Purified monocytes from filaria-infected patients have recently been shown to function less well, based on measurement of phagocytosis or spreading in response to a bacterial stimulus (24).
Infection of a single individual with multiple species of filariae is common, particularly in Africa. For example, the distribution of Mansonella perstans infection, generally nonpathogenic, often overlaps with that of W. bancrofti infection (1, 12, 30). We have previously shown with this same group of patients (12) that ivermectin-albendazole treatment alters only the levels of W. bancrofti MF and circulating filarial antigen and does nothing to the levels of M. perstans MF.
Our objective in this study was to examine the function of monocytes directly ex vivo in a region of the world where filarial infection is endemic and to see if the function of these cells is altered following ivermectin-albendazole administration.
MATERIALS AND METHODS
Study population. (i) Pretreatment.
One hundred fifty individuals residing in Sabougou (in the Koulikoro District of Mali, located outside the coverage area for the Onchocerciasis Control Program) were screened with a questionnaire regarding symptoms of lymphatic filariasis and the types of symptoms commonly associated with posttreatment reactions. An immunochromatographic card test for circulating filarial antigen (Binax, Portland, ME) using whole blood was performed in the field; each was read at exactly 10 min according to the manufacturer's instructions. For the purpose of analysis, W. bancrofti infection was based on positivity with a semiquantitative antigen detection kit (TropBio, Townsville, Queensland, Australia). MF counts were done using calibrated thick smears for both W. bancrofti and M. perstans between 9 p.m. and midnight as previously described (12). Seven circulating-antigen-positive and four circulating-antigen-negative individuals were selected for this study (Table 1).
TABLE 1.
Circulating antigen and filarial worm counts in infected patients pre- and posttreatment
| Patient no. | Diagnosisa | TropBio ELISAb, U/ml
|
M. perstans, MF/ml
|
W. bancrofti, MF/ml
|
|||
|---|---|---|---|---|---|---|---|
| Pretreatment | Posttreatment | Pretreatment | Posttreatment | Pretreatment | Posttreatment | ||
| 1 | WB+/MP+ | 6,855 | 6,444 | 350 | 433 | <17 | <17 |
| 2 | WB+/MP+ | 466 | 322 | 517 | 267 | <17 | <17 |
| 3 | WB+/MP+ | 17,747 | NAc | 1,200 | NA | 233 | NA |
| 4 | WB+/MP+ | 13,852 | 3,336 | 100 | 50 | 617 | <17 |
| 5 | WB+/MP− | 1,377 | 68 | <17d | <17 | <17 | <17 |
| 6 | WB+/MP− | 7,495 | 4,310 | <17 | <17 | <17 | <17 |
| 7 | WB+/MP+ | 21,327 | 9,834 | 50 | <17 | <17 | <17 |
| 8 | WB−/MP+ | <10 | <10 | 283 | 733 | <17 | <17 |
| 9 | WB−/MP+ | <10 | <10 | 1,300 | 1,900 | <17 | <17 |
| 10 | WB−/MP+ | <10 | <10 | 17 | 33 | <17 | <17 |
| 11 | WB−/MP+ | <10 | <10 | 267 | 133 | <17 | <17 |
WB, W. bancrofti; MP, M. perstans.
ELISA, enzyme-linked immunosorbent assay.
NA, not available.
Based on calibrated smear of 70/μl.
All study participants signed informed consent forms, and all protocols were approved by the institutional review boards at both the NIAID and the University of Mali (Bamako).
(ii) Posttreatment.
Individuals were administered ivermectin (100 μg/kg) and albendazole (400 mg) as described previously (12). Posttreatment ex vivo assessments were performed 8 months after the first drug administration (Table 1).
PBMC and monocyte separation.
Peripheral blood mononuclear cells (PBMC) from the blood of Malian volunteers or blood bank donors were isolated by Ficoll diatrizoate density centrifugation (4). Monocytes were isolated from PBMC by using a CD14-negative monocyte isolation kit (Dynal Biotech, Lake Success, NY). Typically, the monocytes were ≥96% pure as assessed by flow cytometry.
Immunofluorescent staining of monocytes.
Monocytes were spun for 5 min at 200,000 cells/slide, using a cytospin, and fixed with methanol-acetone (1:1) for 15 min. After the slides were dry, they were stained with polyclonal rabbit anti-B. malayi MF antibody or control rabbit sera for 1 h. Following a phosphate-buffered saline (PBS) wash, an Alexa-conjugated (Molecular Probes, Eugene, OR) anti-rabbit immunoglobulin G was used at a final concentration of 1:400. The slides were then washed three times with PBS and stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO) at a final concentration of 1 μg/ml for nuclear staining. Following PBS and water rinses, the images were collected on a TCS-SP1 confocal microscope (Leica Microsystems, Exton, PA) using a 63× oil immersion objective.
The polyclonal anti-MF antibody used for this study was raised against B. malayi MF. This antibody has been shown to cross-react with antigens derived from each of the filarial species tested to date.
In vitro activation of monocytes.
Monocytes were separated from PBMC and cultured at 0.5 × 106/ml in a 48-well tissue culture plate in medium alone or activated with Staphylococcus aureus Cowan I bacteria (SAC) (10 μg/ml) plus IFN-γ (1 ng/ml) (SAC/IFN-γ). Culture supernatants were collected and assessed for cytokines.
Cytokine measurement.
All cytokines were detected in culture supernatants by using Searchlight proteome arrays from Pierce Biotechnology (Boston, MA). The sensitivity of detection for IL-1α, MIP-3β, Exodus II, IL-8, and RANTES was 0.4 pg/ml; that for IL-10 and IFN-γ was 0.8 pg/ml; that for IL-12p40 and p70 was 1.2 pg/ml; that for eotaxin was 3.2 pg/ml; and that for transforming growth factor β (TGF-β) was 4.9 pg/ml.
RNA preparation.
Monocytes were separated, and total RNA was prepared using an RNeasy minikit (QIAGEN, Valencia, CA). RNAs from cells obtained from six infected patients and two uninfected healthy individuals for pretreatment studies and from four W. bancrofti-positive patients both pre- and posttreatment were used to make cRNA for microarray analysis.
Microarray analysis.
Total RNA was used to generate cRNA probes. Preparation of cRNA, hybridization, and scanning of the U133A arrays were performed according to the manufacturer's protocol (Affymetrix, Santa Clara, CA). Briefly, 2 to 4 μg of RNA was converted into double-stranded cDNA by reverse transcription using a cDNA synthesis kit (SuperScript Choice; Life Technologies, Gaithersburg, MD) with an oligo(dT)24 primer containing a T7 RNA polymerase promoter site added 3′ of poly(T) (Genset Oligos, La Jolla, CA). After second-strand synthesis, biotin-labeled cRNA was generated from the cDNA sample by an in vitro transcription reaction supplemented with the Bioarray high-yield RNA transcription labeling kit (Enzo, Farmingdale, NY). The biotin-labeled cRNA was purified using RNeasy spin columns (QIAGEN). The labeled cRNA samples were fragmented at 94°C prior to hybridization. Labeled cRNA was hybridized to the U133A microarray while rotating at 60 rpm for approximately 16 h at 45°C. After hybridization, the microarray was washed using the Affymetrix Fluidics Station in buffer containing biotinylated antistreptavidin antibody (Vector Laboratories, Burlingame, CA) for 10 min at 25°C and stained with streptavidin phycoerythrin (final concentration, 10 μg/ml; Molecular Probes) for 10 min at 25°C. Subsequently, the microarray was washed, restained with streptavidin phycoerythrin (10 min, 25°C), and washed again before the fluorescence bound to the microarray was measured at 570 nm in an Affymetrix scanner.
Microarray data processing.
Images of scanned Affymetrix GeneChips were processed using the software and parameter settings suggested by the manufacturer (Affymetrix). The processed fluorescence values were placed into an Excel table (Microsoft Excel Analysis Tools, Seattle, WA) in which rows represented genes and columns represented experimental conditions. We performed statistical analysis using Madb (NCI/CIT mAdb [MicroArray Database] System; http://madb.nci.nih.gov/) and Significance Analysis of Microarray (SAM) (29) for all microarray data. For the pretreatment microarrays, we filtered the data requiring both an unpaired pooled P value of <0.001 and a positive or negative difference (average intensity for infected individuals/average intensity for healthy individuals) in log ratio of greater or less than 2.0 units (requirement equivalent to a fourfold increase or decrease in expression). The selected genes were then applied to SAM using Δ = 0.2 and 0 = number of genes having a false call, giving a final list of genes.
For post- versus pretreatment, the data were filtered based on a paired t test that required a P value of <0.001 as well as a positive or negative difference (the average intensity for the infected individuals posttreatment/pretreatment) in log ratio of greater than 2.0 units (requirement equivalent to a fourfold increase or decrease in expression). The selected genes were then applied to SAM using Δ = 1.2 and 0 = number of genes having a false call, giving a final list of genes. All data filtering was done on a Microsoft Excel spreadsheet. Clustering was done using a web-based hierarchical clustering program (6). For categorizing genes, a web-based program (http://www.libgenechip.niaid.nih.gov; Laboratory of Immunopathogenesis and Bioinformatics, NIAID, NIH) was used (8).
Real-time reverse transcriptase PCR (RT-PCR).
RNA (1 μg) from a single infected individual (patient 6 from Table 1) was used to generate cDNA and then assessed by standard multiplex TaqMan assays (Applied Biosystems, Foster City, CA). The use of a single individual was required as this was the only patient from which there was sufficient RNA to perform this analysis using pre- and posttreatment samples.
Briefly, random hexamers were used to prime RNA samples for reverse transcription using MultiScribe reverse transcriptase, after which PCR products for HSPA4, HSPA8, HSPCA, and HSPCB as well as an endogenous 18S rRNA control were assessed in triplicate wells using TaqMan predeveloped assay reagents. These include PCR primers (forward and reverse) and a 6-carboxyfluorescin dye-labeled TaqMan minor groove binder probe, which cross intron/exon boundaries to amplify only mRNA. For endogenous control a set of primers and a VIC-labeled minor groove binder 18S probe were used. Real-time quantitative RT-PCR was performed on an ABI 7700 sequence detection system (Applied Biosystems).
Statistical analysis.
For pretreatment analyses, the nonparametric Mann-Whitney U test was used, and to compare pretreatment with posttreatment samples, the nonparametric Wilcoxon signed rank test was used. All statistical analyses were performed with StatView 5 (SAS Institute, Cary, NC).
Microarray data accession number.
The data discussed in this paper have been deposited in the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE2135.
RESULTS
Study population.
Of 11 filaria-infected individuals assessed in this report, 7 were positive for circulating W. bancrofti antigen (W. bancrofti positive); 5 of the 7 were found to be also microfilaremic with M. perstans (M. perstans positive). MF counts for W. bancrofti were undetectable in all but two individuals, and those for M. perstans ranged from undetectable (<17) to 1,200 MF/ml (Table 1). The remaining 4 of 11 individuals had no W. bancrofti MF (W. bancrofti negative) in nocturnal blood films but were positive for M. perstans. MF counts in these four individuals ranged from 17 to 1,300 MF/ml (Table 1). Monocytes from each of these individuals were isolated and compared with those of North American uninfected healthy individuals.
Monocytes from filaria-infected individuals are laden with filarial antigens.
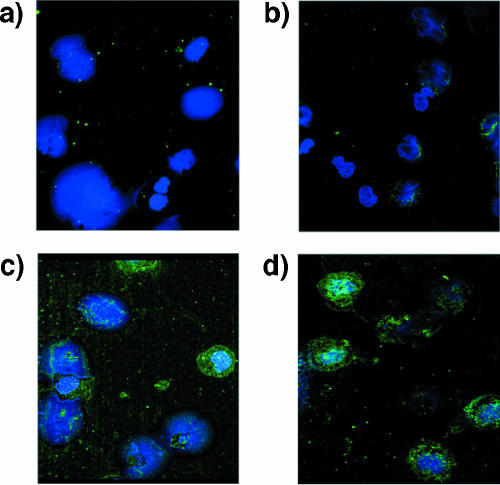
Monocytes from North American blood bank donors and filaria-infected patients were isolated ex vivo pretreatment, and slides were stained with polyclonal anti-MF antibody and DAPI (Fig. 1). As seen, monocytes from filaria-infected patients have internalized antigens to a great degree. This staining of the filarial antigens can be observed in most, but not all, monocytes (Fig. 1). It should be noted, however, that, on the basis of the staining, we cannot distinguish between antigens derived from W. bancrofti and M. perstans (see Materials and Methods).
FIG. 1.
Monocytes from filaria-infected individuals are laden with filarial antigens. Confocal microscopy of monocytes from a W. bancrofti-positive, M. perstans positive individual (a, c, and d) or from a North American healthy donor (b) are shown. (a) Negative control, polyclonal rabbit control antibody. (b, c, and d) Anti-MF rabbit polyclonal antibody. (c) and (d) are from two different regions of the same slide. Green is Alexa staining and blue is DAPI staining of the nuclei of the cells. Objective, 63×.
Monocytes from filaria-infected individuals produce less IL-8, Exodus II, MIP-1α, MIP-1β, and IL-1α than do uninfected normal monocytes.
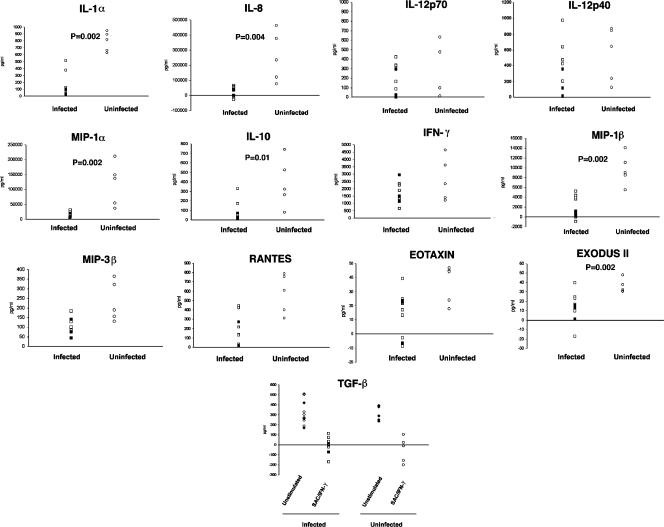
Having identified filarial antigens within the monocyte population, we next tested whether monocytes from filaria-infected individuals function differently than monocytes from the uninfected individuals by assessing the production of monocyte-derived cytokines and chemokines produced either spontaneously (directly ex vivo) or in response to SAC/IFN-γ (Fig. 2). We have previously shown that monocyte-derived DC that are exposed to either live MF or antigens of B. malayi produce less IL-12 and IL-10 in response to SAC/IFN-γ activation (26, 27). Therefore, we used the same stimuli to compare the responses of filaria-infected monocytes with those of normal monocytes. We did not detect a significant difference between the groups in the spontaneous production of any of the cytokines/chemokines tested (data not shown), although in response to SAC/IFN-γ there was a general reduction in the production of many of the measured cytokines/chemokines in the monocytes of filaria-infected individuals compared with those from uninfected North American healthy individuals (Fig. 2). In particular, production of the proinflammatory chemokines (IL-8, Exodus II, MIP-α, and MIP-1β) and the cytokines IL-1α and IL-10 was significantly reduced in filaria-infected individuals. Of interest, the production of TGF-β was not changed in filaria-infected individuals; however, this cytokine was inhibited after activation with SAC/IFN-γ (although not significantly) in both the infected and uninfected groups (Fig. 2, bottom panel).
FIG. 2.
Monocytes from filaria-infected individuals produce less IL-8, Exodus II, MIP-1α, MIP-1β, and IL-1α than monocytes from uninfected individuals. Cytokine production from SAC/IFN-γ-activated monocytes in 10 filaria-infected patients compared with 5 normal monocytes is shown. □, W. bancrofti-positive patients (patients 1 to 7); ▪, W. bancrofti-negative patients (patients 8, 9, and 11); ○, healthy uninfected donors. The value for spontaneous production (medium) has been subtracted from the activated (SAC/IFN-γ) value for all cytokines.
Gene expression comparison of filaria-infected and uninfected monocytes.
Having demonstrated that there is a general suppression of chemokine/cytokine production by monocytes from filaria-infected patients compared with those from healthy uninfected individuals, we assessed more globally the difference between monocytes (ex vivo, unactivated) from infected and uninfected donors by using microarray analysis (Table 2; see Table S1 in the supplemental material). Although we had a small sample size, we used highly conservative constraints for significance (see Materials and Methods). Based on these analyses, the expression of only 62 genes (8 repressed and 54 induced in W. bancrofti-infected individuals) was significantly different in monocytes of W. bancrofti-infected versus W. bancrofti-uninfected individuals (see Table S1 in the supplemental material). When categorized (using Gene Ontology Consortium categories [8]), these genes fell into the following groups based on presumed function: cell growth and/or maintenance, metabolism, response to external stimulus, immune response, signal transduction (e.g., IL-1β, lymphocyte cytosolic protein 2, nuclear receptor subfamily 2, pre-B-cell colony-enhancing factor 1, PDE48 [a cyclic AMP-specific phosphodiesterase], and a triggering receptor expressed on myeloid cells 1), cell death (BCLA2A1, epithelial membrane protein 1, tumor necrosis factor alpha-induced protein 3, and modulator of apoptosis 1), cell adhesion (ICAM-1 and CD44), response to stress, regulation of cell proliferation, cell-cell signaling, embryonic development, and organogenesis.
TABLE 2.
Gene expression comparison of filaria-infected and uninfected monocytesa
| Categoryb and gene product name | Gene product description | P value | Difference |
|---|---|---|---|
| Signal transduction | |||
| NR4A2 | Nuclear receptor subfamily 4, group A, member 2 | 0.000198 | 3.66 |
| PBEF1 | Pre-B-cell colony-enhancing factor 1 | 8.21E−6 | 2.82 |
| PDE4B | Phosphodiesterase 4B, cyclic AMP specific (phosphodiesterase E4 dunce homolog, Drosophila) | 3.45E−6 | 3.12 |
| TREM1 | Triggering receptor expressed on myeloid cells 1 | 0.000929 | 2.85 |
| LCP2 | Lymphocyte cytosolic protein 2 (SH2 domain containing leukocyte protein of 76 kDa) | 0.000551 | 2.76 |
| IL-1β | Interleukin-1β | 0.000361 | 4.11 |
| Cell death | |||
| EMP1 | Epithelial membrane protein 1 | 0.000478 | 3.94 |
| MOAP1 | Modulator of apoptosis 1 | 0.000131 | 3.05 |
| TNFAIP3 | Tumor necrosis factor alpha-induced protein 3 | 0.000399 | 2.31 |
| IL-1β | Interleukin-1β | 0.000361 | 2.76 |
| BCL2A1 | BCL2-related protein A1 | 0.000583 | 4.6 |
| Adhesion | |||
| CD54 (ICAM-1) | Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor | 1.95E−5 | 2.71 |
| CD44 | CD44 antigen (homing function and Indian blood group system) | 9.75E−5 | 2.82 |
Microarray analysis was performed on monocytes from six filaria-infected patients (patients 2, 5, 6, 7, 8, and 10) and compared with that for two uninfected normal monocytes. All monocytes were collected ex vivo and were unactivated. Genes were selected based on statistical analysis using an unpaired t test with a P value of <0.001, a difference (average log intensity of infected individuals/average log intensity of healthy individuals) of 2.0, and a Δ value of 0.2 (see Materials and Methods). The genes listed here represent some of the genes induced in filaria-infected monocytes compared with monocytes from uninfected subjects.
Categorized by Gene Ontology Consortium categories.
Treatment with single-dose ivermectin plus albendazole results in a significant reversal of cytokine inhibition in the monocytes of W. bancrofti-infected patients.
All filaria-infected individuals were treated with single-dose ivermectin plus albendazole; at 8 months posttreatment, the monocytes from the W. bancrofti-infected patients were isolated and compared with those pretreatment. With a larger sample size, we have previously (12) reported that circulating filarial antigen levels at 8 months following treatment were significantly lower than those pretreatment (n = 26; P = 0.010). In addition, a significant decrease in W. bancrofti MF counts was reported (n = 15; P = 0.002); however, in the same study, it was shown that M. perstans counts did not change significantly (n = 29; P = 0.58) following treatment (12) (Table 1). As the present study utilized only a subset of these patients, only one of whom was W. bancrofti MF positive, inferences of statistical significance for W. bancrofti MF counts cannot be made. Also, some filarial antigens (most likely M. perstans antigens) could still be detected in monocytes at 8 months following treatment when immunofluorescent staining was performed (data not shown).
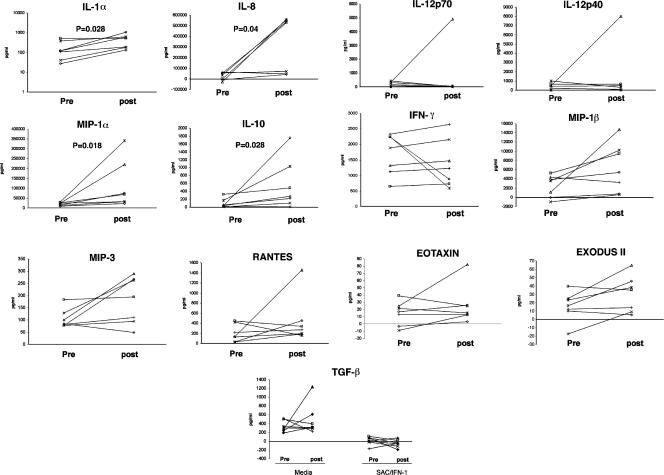
In general, the production of most cytokines/chemokines tested posttreatment in response to SAC/IFN-γ was greater than the corresponding levels pretreatment. Although there was not a significant change in basal-level production of these analytes pre- versus posttreatment, in response to activation by SAC/IFN-γ, production of IL-8 (P = 0.04), IL-1α (P = 0.028), MIP-1α (P = 0.018), and IL-10 (P = 0.028) was significantly increased (Fig. 3) following treatment. Furthermore, using Spearman rank correlation analysis, there was no relationship between the reduction in the level of circulating antigen and the change (pre- versus posttreatment) in production of IL-1α, IL-8, MIP-1α, and IL-10 following treatment.
FIG. 3.
Treatment with single-dose ivermectin plus albendazole results in a significant induction of IL-8, IL-1α, MIP-1α, and IL-10 in the monocytes of W. bancrofti-positive patients. Cytokine production from SAC/IFN-γ-activated monocytes pre- versus posttreatment for seven W. bancrofti-positive patients (patients 1 to 7) is shown. The value for spontaneous production (medium) is subtracted from the activated (SAC/IFN-γ) value for all analytes.
Treatment with single-dose ivermectin plus albendazole alters monocyte gene expression at 8 months.
Microarray analysis using RNA prepared immediately ex vivo from monocytes of W. bancrofti-positive individuals pretreatment and 8 months following ivermectin-albendazole administration demonstrated that 47 genes were repressed and 41 genes were induced in paired (pre- versus posttreatment; see Table S2 in the supplemental material) analysis. Utilizing hierarchical clustering, we could easily find distinct sets of genes that were highly expressed pretreatment and repressed following treatment, as well as the converse (see Fig. S1 in the supplemental material). Genes induced posttreatment fell into several categories (Table 3; see Table S2 in the supplemental material), but the most striking were the many members of the heat shock protein (HSP) family with ATP binding activity (HSPA8 [70 kDa], HSPCA [protein 1α; 90 kDa], HSPH1 [105 kDa/110 kDa], HSPA4 [70 kDa], HSPCB [protein 1β; 90 kDa], HSPD1 [60 kDa], HSPE1 [10 kDa], T-complex 1 [TCP1]; and chaperonin-containing TCP1, subunit 3γ [CCT3], with a chaperone activity). In addition, BCL2-associated athanogene 3, involved in Hsp70/Hsc70 protein regulator activity, and suppression of tumorigenicity 13, involved in Hsp70/Hsp90 organizing protein activity, as well as DNAJB1 (Hsp40 homolog), were also induced posttreatment.
TABLE 3.
Gene expression in W. bancrofti-positive individuals pre- and posttreatmenta
| Categoryb and gene product name | Gene product description | P value | Difference |
|---|---|---|---|
| Genes induced posttreatment | |||
| Signal transduction | |||
| DPYSLY2 | Dihydropyrimidinase-like 2 | 0.005252 | 4.12 |
| GEM | GTP binding protein overexpressed in skeletal muscle | 0.000299 | 2.26 |
| SCAP2 | Src family-associated phosphoprotein 2 | 0.007323 | 2.02 |
| TEBP | Unactive progesterone receptor, 23 kDa | 0.000492 | 2.48 |
| ADCY2.51 | Adenylase cyclase 7 | 0.003234 | 2.51 |
| IL-1RI5.03 | Interleukin-1 receptor, type I | 0.008116 | 5.03 |
| Protein metabolism | |||
| BAG3 | BCL2-associated athanogene 3 | 0.009291 | 3.35 |
| CCT3 | Chaperonin-containing TCP1, subunit 3 (gamma) | 0.007919 | 2.14 |
| DNAJB1 | DnaJ (Hsp40) homolog, subfamily B, member 1 | 0.008126 | 4.79 |
| HSPA4 | Heat shock 70 kDa protein 4 | 0.005675 | 2.38 |
| HSPA8 | Heat shock 70-kDa protein 8 | 0.000704 | 2.54 |
| HSPA8 | Heat shock 70-kDa protein 8 | 0.006557 | 3.61 |
| HSPCA | Heat shock 90-kDa protein 1, alpha | 0.000784 | 3.49 |
| HSPCB | Heat shock 90-kDa protein 1, beta | 0.023143 | 2.57 |
| HSPCB | Heat shock 90-kDa protein 1, beta | 0.010146 | 3.76 |
| HSPD1 | Heat shock 60-kDa protein 1 (chaperonin) | 0.001577 | 2.34 |
| HSPE1 | Heat shock 10-kDa protein 1 (chaperonin 10) | 0.005329 | 4.47 |
| HSPH1 | Heat shock 105-kDa/110-kDa protein 1 | 0.000629 | 5.17 |
| ST13 | Suppression of tumorigenicity 13 (colon carcinoma) (Hsp70 interacting protein) | 0.010874 | 2.42 |
| TCP1 | T-complex 1 | 0.000148 | 2.73 |
| Genes repressed posttreatment | |||
| Signal transduction | |||
| ARL7 | ADP-ribosylation factor-like 7 | 0.002343 | −3.29 |
| CCR7 | Chemokine (C-C motif) receptor 7 | 0.012843 | −2.16 |
| EBI2 | Epstein-Barr virus-induced gene 2 (lymphocyte-specific G protein-coupled receptor) | 0.0062 | −2.57 |
| MAP3K8 | Mitogen-activated protein kinase 8 | 0.000245 | −2.48 |
| SPHK1 | Sphingosine kinase 1 | 0.000581 | −2.32 |
| Protein metabolism | |||
| PPID | Peptidylprolyl isomerase D (cyclophilin D) | 0.004214 | −3.91 |
| MAP3K8 | Mitogen-activated protein kinase 8 | 0.000245 | −2.48 |
| CTSL | Cathepsin L | 0.004541 | −3.04 |
| PPIF | Peptidylprolyl isomerase F (cyclophilin F) | 4.90E−5 | −2.38 |
| TGIF | TGFβ-induced factor (TALE family homeobox) | 0.000353645 | −2.85 |
| TGFB1I4 | Transforming growth factor β1-induced transcript 4 | 0.002675247 | −2.32 |
Microarray analysis was performed on monocytes from four W. bancrofti-positive patients (patients 2, 5, 6, and 7) pretreatment and compared with that for the same patients' monocytes at 8 months following treatment with ivermectin-albendazole. Genes were selected based on statistical analysis using a paired t test with a P value of <0.001, a difference (average log intensity of infected individuals posttreatment/average intensity of infected individuals pretreatment) of 2.0, and a Δ value of 1.2 (see Materials and Methods). Positive differences represent genes induced posttreatment, and negative differences represent genes repressed posttreatment.
Categorized by Gene Ontology Consortium categories.
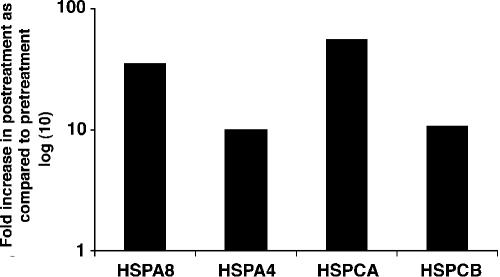
To confirm and better quantify the posttreatment induction of these HSPs, real-time quantitative RT-PCR was performed using primers and probes (as described in Materials and Methods) specific for HSPA4, HSPA8, HSPCA, and HSPCB and RNA from monocytes obtained pre- and posttreatment. The posttreatment increase in expression of each of these genes ranged from 8- to 50-fold (Fig. 4) compared with the analogous pretreatment expression.
FIG. 4.
mRNA expression of heat shock genes is induced in monocytes of an infected patient (patient 6) at 8 months following treatment with ivermectin-albendazole. Heat shock genes in the infected individual posttreatment compared with pretreatment, using real-time RT-PCR, relative to rRNA are shown.
Genes involved in signal transduction, such as those for interleukin-1 receptor type I, unactive progesterone receptor TEBP, dihydropyrimidinase-like 2, adenylase cyclase 7, Src family-associated phosphoprotein 2, and GTP binding protein overexpressed in skeletal muscle, were also expressed at higher levels posttreatment in these individuals (Table 3).
Among the genes repressed in the posttreatment samples (Table 3; see Table S2 in the supplemental material) were those involved in signaling (chemokine receptor 7, mitogen-activated protein kinase 8, Epstein-Barr virus-induced gene 2, and sphingosine kinase 1), transcription (such as transforming growth factor β-induced factor and transforming growth factor β-stimulated protein TSC-22), and protein metabolism (peptidylprolyl isomerase D, cathepsin L, and peptidylprolyl isomerase F) (Table 3). In addition, TGF-β-induced transcript 4 and TGF-β-induced factor, TALE family homeobox, were also repressed following treatment. This is of interest because TFG-β may be involved in the hyporesponsiveness seen in MF patients (13). Whether there is a correlation between these genes and reversal of monocyte dysfunction remains to be studied.
DISCUSSION
Among the many explanations for the downregulated parasite-specific T-cell responses seen in humans with filarial infections, the least well investigated has been the role of APC. As in vivo studies of monocytes in humans were lacking, we chose to investigate the influence of filarial infection on monocytes by isolating and studying these cells immediately ex vivo from filaria-infected individuals. Most interesting was the finding that monocytes from infected patients contained membrane-bound and/or intracytoplasmic filarial antigens. The capacity of antigen internalization has also been shown in vitro (25, 27), but the fact that the monocytes from infected patients are studded with filarial antigens suggests that throughout the course of infection (often lifelong), the monocytes and the cells differentiated from them (e.g., monocyte-derived DC or macrophages) are under the influence of secreted/excreted products of the filarial worms.
In general, the monocytes of filaria-infected patients had a reduced ability to produce chemokines such as MIP-1α, MIP-1β, IL-8, RANTES, MIP-3β, eotaxin, and Exodus II, as well as cytokines such as IL-1α, IL-12p40, IL-12p70, IFN-γ, and IL-10, in response to activation with SAC/IFN-γ compared with monocytes from uninfected individuals, a reduction that has previously been shown to translate into diminished function (25-27). Of interest, it has been shown that while dead or dying worms induce proinflammatory responses in mice, live and healthy worms secrete materials that appear to induce the differentiation of alternatively activated macrophages that downregulate an inflammatory response (reviewed in reference 2). Our results parallel those seen in the animal models, in that monocytes from patently infected individuals have a diminished proinflammatory capacity that may secondarily modulate some immune responses.
One of the problems with the present study (and one that was unable to be addressed in the field setting chosen for this study) was the presence of both W. bancrofti and M. perstans infection within the same host. Clearly, infection with one filarial species could potentially modify the clinical course or response to treatment of another species. Fortunately, because M. perstans is unaffected by single doses of albendazole-ivermectin (12) and because M. perstans MF counts were unchanged 8 months after treatment, the changes noted pre- and posttreatment had to be, by definition, related to W. bancrofti infection only. Of particular interest was the increased production of the same chemokines/cytokines diminished in W. bancrofti circulating-antigen-positive patients pretreatment. In particular, IL-1α, MIP-1α, IL-8, and IL-10 levels were significantly increased posttreatment, suggesting that W. bancrofti infection specifically diminishes the production of these chemokines and cytokines.
In assessing the gene expression comparison between monocytes from filaria-infected patients and from healthy individuals, it was found, surprisingly, that only 62 (of greater than 30,000) genes demonstrated expression differences between infected and uninfected individuals. Among the genes expressed to a greater degree in patients were those for products involved in apoptosis (IL-1β, BCL2A1, and MOAP1) and cell adhesion (CD44 and ICAM-1). The expression of both these sets of genes reiterates previous in vitro findings, in particular those that show that live MF not only induce cell death in monocyte-derived DC but also induce expression of ICAM-1 (26). ICAM-1 has been clearly shown to be important in helminth infection. For example, it has been shown that in Schistosoma mansoni, granuloma formation requires the expression of ICAM-1 (22), and it has been demonstrated that in a model of eye disease in onchocerciasis (“river blindness”), recruitment of eosinophils to the cornea is mediated by ICAM-1 expression on limbal vessels (11); however, the exact role of ICAM-1 in filarial infection is not known and needs further investigation. Indeed, other factors such as genetic background, health status, nutrition, and coinfection may also be involved in the difference seen between the infected individuals and healthy blood bank donors.
Comparing global expression pre- and posttreatment was actually the most instructive. First, the basal gene expression patterns were clearly altered between pre- and posttreatment paired samples. The most intriguing alterations were in the expression of heat shock genes (Hsp60, Hsp70, Hsp90, and Hsp105) (Fig. 4), in which there was a stereotypical response posttreatment.
HSPs possess chaperone activity and are expressed in nonstressed cells at low levels. They have essential functions in the cell cycle, cellular differentiation and growth, metabolism, and apoptosis through protein assembly and can influence the activation of enzymes and receptors. In addition to these functions, HSPs play a role in immune activation and recognition (21). One essential immunoregulatory function described for certain HSPs is activation of the innate immune system (14, 28). It has also been shown that Hsp65 (the chaperonin of Mycobacterium tuberculosis) stimulates human monocytes to release proinflammatory cytokines (9). Bethke et al. (3) have also shown that Hsp60 and, to a lesser extent, Hsp72 induce DC maturation and induce release of proinflammatory cytokines from monocytes and immature DC.
Our posttreatment data indicate not only that gene expression of HSPs is induced in monocytes of W. bancrofti-positive patients but also that these monocytes have elevated levels of proinflammatory chemokines/cytokines that may or may not be the direct effect of the HSPs. One explanation is that treatment with ivermectin-albendazole (which resulted in clearance of circulating MF antigens) reversed the suppressive function of monocytes, which then play an important role in acute inflammatory processes by releasing cytokines, increasing the expression of HSPs to activate antimicrobial function of these cells, and preparing them to confront the parasite.
HSPs can also play a role in programmed cell death (15, 23). In our study, whether the increased expression of HSP genes posttreatment results in resistance of these monocytes to apoptosis is not known. Clearly, in our in vitro data, live MF induced cell death in monocyte-derived DC (26). Whether filaria-infected monocytes in vivo undergo apoptosis awaits clarification.
Our data collectively suggest that filaria-infected patients have impaired monocyte function that is reflected by their inability to produce cytokines/chemokines in response to activating stimuli and by alteration in their basal gene expression (expressing more proapototic genes and adhesion molecules such as ICAM-1). This mechanism of impaired monocyte function is still under investigation and is not well understood. Whether the filarial worms alter antigen processing and presentation generally, resulting in their lack of ability to process and present bystander antigens, is currently under investigation. In this study, 8 months after ivermectin-albendazole treatment, these monocytes can reacquire the ability to produce the same cytokines/chemokines, some of which are involved in the inflammatory response. In addition, the expression of important genes involved in innate immunity and protection against infection (HSP genes) is induced. This induction of HSPs may be important in elevating the production of proinflammatory cytokines, protecting these cells against apoptosis, and preparing the cells to combat subsequent infections.
Supplementary Material
Acknowledgments
We are grateful to the faculty and staff of the Malaria Research and Training Center of the National Institute of Allergy and Infectious Diseases and the University of Mali School of Medicine, Pharmacy, and Dentistry who provided us with laboratory facilities and logistics support. We are also grateful to Y. Sidibè, Chief Medical Officer in Kolokani, and to the health care teams of Kolokani and Massantola for their participation in the study. Most importantly, we thank the residents and leaders of Sabougou, especially the study participants, for their cooperation. For statistical analysis we thank Timothy Meyers, Michael Fay, Esther Asaki, and John Powell. We also thank Jun Yang, Rich Leimpicki, and Damien Chaussabel for their help with microarray studies; Owen Schwartz for confocal microscopy; and Brenda Rae Marshall for editorial help.
This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Bethesda, Maryland) and by the Malaria Research and Training Center of the National Institute of Allergy and Infectious Disease (Bamako, Mali).
Editor: J. F. Urban, Jr.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Agbolade, M., and D. O. Akinboye. 2001. Loa loa and Mansonella perstans infections in Ijebu northwestern Nigeria: a parasitological study. Jpn. J. Infect. Dis. 54:108-110. [PubMed] [Google Scholar]
- 2.Allen, J. E., and P. Loke. 2001. Divergent roles for macrophages in lymphatic filariasis. Parasite Immunol. 23:345-352. [DOI] [PubMed] [Google Scholar]
- 3.Bethke, K., F. Staib, M. Distler, U. Schmitt, H. Jonuleit, A. H. Enk, P. R. Galle, and M. Heike. 2002. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J. Immunol. 169:6141-6148. [DOI] [PubMed] [Google Scholar]
- 4.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. 97:77-89. [PubMed] [Google Scholar]
- 5.Chaussabel, D., R. T. Semnani, M. A. McDowell, D. Sacks, A. Sher, and T. B. Nutman. 2003. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102:672-681. [DOI] [PubMed] [Google Scholar]
- 6.Chaussabel, D., and A. Sher. 2002. Mining microarray expression data by literature profiling. Genome Biol. 3:0055.1-0055.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai, W. J., and B. Gottstein. 1999. Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology 97:107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis, G., B. T. Sherman, D. A. Hosack, J. Yang, W. Gao, H. C. Lane, and R. A. Lempicki. 2003. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 4:P3. [PubMed] [Google Scholar]
- 9.Friedland, J. S., R. Shattock, D. G. Remick, and G. E. Griffin. 1993. Mycobacterial 65-kD heat shock protein induces release of proinflammatory cytokines from human monocytic cells. Clin. Exp. Immunol. 91:58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenson, J. S., R. O'Connor, J. Osborne, and E. Devaney. 2002. Infection with Brugia microfilariae induces apoptosis of CD4+ T lymphocytes: a mechanism of immune unresponsiveness in filariasis. Eur. J. Immunol. 32:858-867. [DOI] [PubMed] [Google Scholar]
- 11.Kaifi, J. T., E. Diaconu, and E. Pearlman. 2001. Distinct roles for PECAM-1, ICAM-1, and VCAM-1 in recruitment of neutrophils and eosinophils to the cornea in ocular onchocerciasis (river blindness). J. Immunol. 166:6795-6801. [DOI] [PubMed] [Google Scholar]
- 12.Keiser, P. B., Y. I. Coulibaly, F. Keita, D. Traaore, A. Diallo, D. A. Diallo, R. T. Semnani, O. K. Doumbo, S. F. Traore, A. D. Klion, and T. B. Nutman. 2003. Clinical characteristics of post-treatment reactions to ivermectin/albendazole for Wuchereria bancrofti in a region co-endemic for Mansonella perstans. Am. J. Trop. Med. Hyg. 69:331-335. [PubMed] [Google Scholar]
- 13.King, C. L., S. Mahanty, V. Kumaraswami, J. S. Abrams, J. Regunathan, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Investig. 92:1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kol, A., T. Bourcier, A. H. Lichtman, and P. Libby. 1999. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Investig. 103:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liossis, S. N., X. Z. Ding, J. G. Kiang, and G. C. Tsokos. 1997. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J. Immunol. 158:5668-5675. [PubMed] [Google Scholar]
- 16.Loke, P., A. S. MacDonald, A. Robb, R. M. Maizels, and J. E. Allen. 2000. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur. J. Immunol. 30:2669-2678. [DOI] [PubMed] [Google Scholar]
- 17.Mahanty, S., H. E. Luke, V. Kumaraswami, P. R. Narayanan, V. Vijayshekaran, and, T. B. Nutman. 1996. Stage-specific induction of cytokines regulates the immune response in lymphatic filariasis. Exp. Parasitol. 84:282-290. [DOI] [PubMed] [Google Scholar]
- 18.Mahanty, S., S. N. Mollis, M. Ravichandran, J. A. Abrams, V. Kumaraswami, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1996. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J. Infect. Dis. 173:769-773. [DOI] [PubMed] [Google Scholar]
- 19.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-744. [DOI] [PubMed] [Google Scholar]
- 20.Nutman, T. B., and V. Kumaraswami. 2001. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 23:389-399. [DOI] [PubMed] [Google Scholar]
- 21.Prohaszka, Z., and G. Fust. 2004. Immunological aspects of heat-shock proteins—the optimum stress of life. Mol. Immunol. 41:29-44. [DOI] [PubMed] [Google Scholar]
- 22.Ritter, D. M., and J. H. McKerrow. 1996. Intercellular adhesion molecule 1 is the major adhesion molecule expressed during schistosome granuloma formation. Infect. Immun. 64:4706-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samali, A., and T. G. Cotter. 1996. Heat shock proteins increase resistance to apoptosis. Exp. Cell Res. 223:163-170. [DOI] [PubMed] [Google Scholar]
- 24.Sasisekhar, B., M. Aparna, D. J. Augustin, P. Kaliraj, S. K. Kar, T. B. Nutman, and R. B. Narayanan. 2005. Diminished monocyte function in microfilaremic patients with lymphatic filariasis and its relationship to altered lymphoproliferative responses. Infect. Immun. 73:3385-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semnani, R. T., M. Law, J. Kubofcik, and T. B. Nutman. 2004. Filaria-induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. J. Immunol. 172:6229-6238. [DOI] [PubMed] [Google Scholar]
- 26.Semnani, R. T., A. Y. Liu, H. Sabzevari, J. Kubofcik, J. Zhou, J. K. Gilden, and T. B. Nutman. 2003. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J. Immunol. 171:1950-1960. [DOI] [PubMed] [Google Scholar]
- 27.Semnani, R. T., H. Sabzevari, R. Iyer, and T. B. Nutman. 2001. Filarial antigens impair the function of human dendritic cells during differentiation. Infect. Immun. 69:5813-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava, P. K., A. Menoret, S. Basu, R. J. Binder, and K. L. McQuade. 1998. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity 8:657-665. [DOI] [PubMed] [Google Scholar]
- 29.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wanji, S., N. Tendongfor, M. Esum, S. Ndindeng, and P. Enyong. 2003. Epidemiology of concomitant infections due to Loa loa, Mansonella perstans, and Onchocerca volvulus in rain forest villages of Cameroon. Med. Microbiol. Immunol. 192:15-21. [DOI] [PubMed] [Google Scholar]
- 31.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453-6460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.