Abstract
Vaccine strategies, such as influenza virus vaccination of the elderly, are highly effective at preventing disease but provide protection for only the responding portion of the vaccinees. Adjuvants improve the magnitude and rates of responses, but their potency must be attenuated to minimize side effects. Topical delivery of strong adjuvants such as heat-labile enterotoxin from Escherichia coli (LT) induces potent immune responses. We hypothesized that LT delivered alone in an immunostimulating (LT-IS) patch placed on the skin at the site of injection could augment the immune response to injected vaccines. This was based on the observation that topically applied LT induces migration of activated antigen-presenting cells (APCs) from the skin to the proximal draining lymph node (DLN), and that APCs loaded with antigen by injection in the same anatomical region also migrate to the same DLN. We observed that when influenza virus vaccine is injected and an LT-IS patch is placed to target the same DLN, the influenza virus antibody response is enhanced. Similarly, influenza virus-specific T cells isolated from the lungs show increased levels of gamma interferon and interleukin-4 production. An LT-IS patch placed near an injected vaccine also leads to increased levels of hemagglutination inhibition titers, enhanced mucosal immunoglobulin A responses, and enhanced antigen presentation. Although the mechanisms by which an LT-IS patch exerts its enhancing effects need further study, the enhanced immune responses, ability to safely use potent adjuvants, and simplicity of LT-IS patch application address an important unmet need and provide a new immune enhancement strategy.
The skin immune system has recently been recognized as a highly attractive target for vaccine delivery (17). This is in part driven by characterization of skin immune mechanisms (20) and in part by renewed interest in needle-free vaccine delivery strategies (10). Transcutaneous immunization (TCI) is a novel, needle-free approach to immunization using a patch or similar means to deliver vaccine antigens with accompanying adjuvants via the skin, and it is in clinical evaluation (12, 15). The immunostimulation achieved by the use of adjuvants in the skin appears to take advantage of the potent antigen-presenting cells (APCs) in the epidermis, Langerhans cells (LCs), which in combination with topical use of the most powerful adjuvants lead to strong systemic immune responses to vaccines.
Adjuvants may play their greatest role with poorly immunogenic vaccine antigens or in settings of immune compromise such as the senescent immune system (9, 33). The notable immune stimulation observed by the use of adjuvants on the skin suggested that topical delivery of adjuvants to the skin might be used to augment immune responses to vaccines delivered concurrently by other routes, i.e., using a patch at the time of injection. Influenza virus vaccination of the elderly, while effective, falls well short of fully protecting the recipients due to the low response rates to the vaccine. Even a modest enhancement of the immune response could lead to significant impact on morbidity and mortality (6, 24). In principle, the use of coinjected adjuvants in the vaccine has been shown to modestly but significantly enhance influenza virus vaccine immune responses in the elderly (9). However, the immune enhancement achieved by the use of adjuvants is often accompanied by side effects (9, 24, 29). The adjuvant MF59, used in the influenza virus vaccine targeting the elderly, has been modified to decrease the reactogenicity, achieving a balance between reactogenicity and potency (13). The use of heat-labile enterotoxin from Escherichia coli (LT) as an adjuvant is attractive, as LT is potent, can readily be delivered to the human epidermis, and has been safely used on the skin in several clinical trials (12, 15).
LCs are bone marrow-derived dendritic cells (DCs) residing in the epidermis. They play a dual role of immunosurveillance in the skin and, upon activation by microorganisms or their products, crawl out of the skin to the draining lymph nodes (DLNs), where they orchestrate a specific immune response (2, 22). Activated, mature LCs are APCs, express high levels of costimulatory molecules, and secrete cytokines, resulting in strong immune effector responses by B and T lymphocytes (2, 34).
We hypothesized that adjuvant-activated epidermal LCs could exert bystander or direct immunostimulatory effects on APCs loaded with antigen by injection if they targeted the same DLN, leading to enhanced immune responses to injected vaccines. We and others have found that stimulation of LCs in the skin leads to increased populations of activated DCs in the DLN (1, 17, 22, 34). In the present study, we show that application of an immunostimulating patch containing LT (LT-IS patch) on the skin over the same DLN field as an injected influenza virus vaccine leads to markedly enhanced serum influenza virus-specific antibody responses. We also show that an LT-IS patch leads to increased levels of functional antibody responses, enhanced T-cell responses, and enhanced antigen presentation. While the mechanisms by which this may occur remain to be fully defined, the enhanced immune responses, ability to safely use potent adjuvants, and simplicity of the LT-IS patch application may lead to a new concept for improving the effectiveness of immunizations.
MATERIALS AND METHODS
Immunization.
Female C57BL/6 mice (n = 5 to 8) 6 to 8 weeks of age were obtained from Taconic Laboratories. (i) Subcutaneous (s.c.), intradermal (i.d.), and intramuscular (i.m.) immunizations: mice were immunized either s.c. or i.d. under the dorsal caudal surface at the base of the tail or i.m. in the right and left femoral muscles with 5 μg of trivalent influenza split virus vaccine (influenza virus vaccine) containing A/Panama/2007, A/New Caledonia/20/99, and B/Johannesburg/15/99 strains (Aventis Pasteur, Lyon, France). (ii) Transcutaneous immunizations: mice were immunized on the skin as described previously (32). Briefly, shaved skin of anesthetized mice was pretreated by hydration with saline-soaked gauze; the stratum corneum was disrupted by mild abrasion with emery paper (GE Medical Systems). Wet patches containing phosphate-buffered saline (PBS) or LT (Berna Biotech, Switzerland) were applied on pretreated skin for 1 h or overnight.
Lung wash and cell isolation.
Lung washes were collected as described previously (11). Lungs were perfused by flushing the right ventricle of the heart with PBS. The organs were removed, minced, and dissociated by incubation in digestion medium (10% fetal bovine serum [Life Technologies], 125 U of collagenase/ml, 60 U of hyaluronidase/ml, 60 U of DNase I [Sigma]/ml) for 1 h at 37°C and constant agitation. Homogenates were filtered, treated with ACK lysing buffer (Life Technologies), and washed.
ELISA.
Influenza virus- and LT-specific immunoglobulin G (IgG), IgG1, IgG2a, and IgA titers were determined in sera and lung washes by enzyme-linked immunosorbent assay (ELISA) on 96-well plates (Immulon-2HB; Dynex Laboratories) coated overnight with each strain of influenza split virus, as described elsewhere (11). Antibody titers are reported as the optical density at 405 nm (OD405) or ELISA units, which correspond to the inverse dilution of the serum that yielded an OD405 of 1.0.
HAI titers.
Serum samples from days 0 and 52 postimmunization were analyzed by John Treanor at the University of Rochester for hemagglutination inhibition (HAI) titers as described previously (16).
ELISPOT.
ELISPOT was used to measure the number of cells producing interleukin-4 (IL-4) and gamma interferon (IFN-γ). Multiscreen-HA membrane plates (Millipore) were coated with monoclonal antibodies specific for mouse IFN-γ (Biosource) and IL-4 (Pharmingen), stored overnight at 4°C, and blocked with 1% bovine serum albumin (BSA) in PBS for 1 h at 37°C. Cells isolated from lungs were plated starting at 106/well in duplicate and serially diluted. Cells were cultured overnight in the presence of influenza split virus dialyzed against PBS or medium alone. Spots were resolved as described previously (28).
Antibodies and fluorescent dyes.
Antibodies (BD Biosciences) specific for mouse CD11c (biotin, phycoerythrin [PE], and APC), CD16/32, CD19 (PerCP-Cy5), CD86 (PE), and H-2 IAb (PE) were used at 5 μg/ml, and streptavidin-CyChrome was used at 0.1 μg/ml. Fluorescein isothiocyanate (FITC) (Sigma) was reconstituted at 100 mg/ml in dimethyl sulfoxide and then diluted into PBS. A viability dye (ethidium homodimer) was used to stain dead cells that were gated out of all flow cytometry graphs. Cells were suspended in ice-cold PBS supplemented with 1% BSA and incubated with Fc Block (CD16/32) for 20 min before addition of 5 μg of fluorescent antibodies/ml and viability dye. After 30 min, cells were washed twice with ice-cold 1% BSA in PBS and analyzed using a FACSCalibur (BD Biosciences).
LC labeling and analysis.
Skin at the dorsal caudal surface was painted with FITC alone in PBS or admixed with LT. Twenty-four hours later, DLNs (inguinal) were isolated and stained for CD11c, H-2 IAb, and CD86 (Pharmingen).
Fluorescent protein conjugates.
Ovalbumin (OVA; Sigma) and LT were conjugated to succinimidyl esters of AlexaFluor (Molecular Probes)-reactive dyes. OVA was conjugated to either AlexaFluor 488 or AlexaFluor 633. LT was conjugated to AlexaFluor 488. Briefly, 0.9 ml of a 15-mg/ml OVA solution or a 6-mg/ml LT solution in PBS plus 0.1 M sodium bicarbonate and 1 mg of AlexaFluor dye reconstituted with 25 μl of dimethyl sulfoxide were combined and incubated at room temperature with constant stirring for 4 h. Protein conjugated with dye was separated from free dye by size-exclusion chromatography using a 6-cm by 1.5-cm Sepahcryl S-200 (Amersham Pharmacia Biotech AB) column and PBS running buffer. Conjugated protein was concentrated using a Centricon 30 (Amicon). After conjugation, LT maintained monosialoganglioside GM1 receptor binding activity (data not shown) and 20-fold lower in vitro ADP-ribosyltransferase activity (data not shown). DQ-OVA (Molecular Probes) is a quenched fluorescent dye that fluoresces only after proteolysis, releasing two fragments with fluorescence excitation and emission maxima of 505 and 515 nm, respectively.
RESULTS
LT induces epidermal LC migration to the DLNs.
The role of LCs in the afferent arm of immune response induction is well established (20, 22). Consistent with these findings, intradermally injected West Nile live virus, plasmid DNA with encoded adjuvants, and topical cholera toxin increase the migration of LCs out of the skin and into the DLNs (1, 17, 21). As the robust immune responses that follow TCI depend on the use of potent adjuvants such as LT (15, 32), we hypothesized that LT activates LCs, which then migrate to the DLN for antigen presentation (12, 17). To address the effect of LT on migration of epidermal LCs, we used an aqueous solution of FITC to track LCs, which minimized the activating effect of acetone-solubilized FITC (17, 22). Following topical FITC-LT application, the number of FITC+ cells increased in the DLN (Fig. 1C), and the FITC+ cells derived from LT-treated skin demonstrated a strong up-regulation of CD86 and modest increase in major histocompatibility complex (MHC) class II (Fig. 1F and I). Similar results were seen in studies with direct labels of the adjuvant or antigen (data not shown; see also Fig. 7, below), suggesting that LT coadministered with antigen promotes the activation, maturation, and migration of activated LCs from skin to DLNs.
FIG. 1.
LT activates LC migration to DLNs. Mice (n = 3) were topically treated with FITC (B, E, and H) or FITC admixed with LT (C, F, and I). Inguinal LN cells were isolated 24 h later from naïve (A, D, and G) or immunized mice and incubated with fluorescent dye-conjugated antibodies. Large granular cells were gated and analyzed for surface expression of CD11c (A to C), MHC class II (D to F), and CD86 (G to I). The percentage of double-positive cells is listed in the upper right quadrant of each dot plot. To determine the migration and distribution of LCs from the skin to the LN, inguinal (J), axillary (K), and superficial (L) cervical LNs were isolated from mice treated with FITC admixed with LT, stained for CD11c, and analyzed for the amount of FITC uptake. The percentage of CD11c+ FITC+ cells is listed in panels J to L. Cells from naïve mice were used to set the background fluorescence and the marker.
FIG. 7.
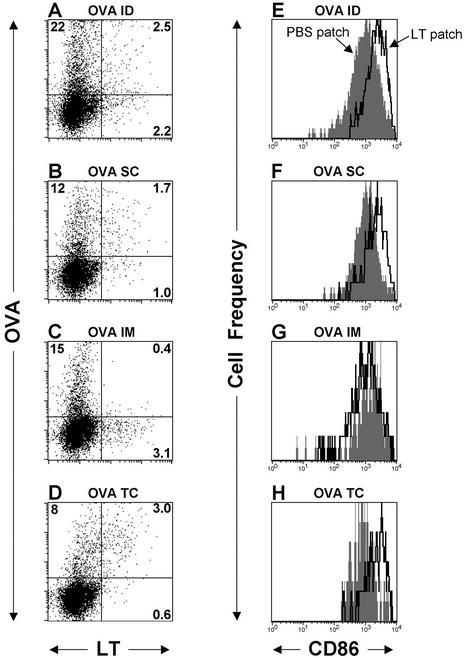
Migration and activation marker up-regulation of CD11c+ dendritic cells after application of an LT-IS patch. Mice (n = 3) were immunized with 50 μg of AlexaFluor 633-conjugated OVA (AF-OVA) by i.d. (A and E), s.c. (B and F), or i.m. (C and G) injection, or with 300 μg of AF-OVA by topical application (D and H) followed by a 30-μg AlexaFluor 488-conjugated LT-IS patch (A to D) or a 40-μg LT patch (E to H). After 20 h, inguinal (draining) LNs were excised, processed, and stained with fluorescently labeled antibodies. Panels A to D were gated on large, granular, CD11c+, CD19− live cells, and panels E and F were gated on large, granular, CD11c+, CD19−, OVA+ live cells. In panels E to H, the histogram overlays compare the levels of CD86 on gated cells from mice immunized with a PBS (closed gray histogram) or LT (open black lined histogram) patch, as indicated by arrows. The percentage of CD11c+ cells is listed in quadrants A to D.
Adjuvant-activated APCs from the skin target DLNs.
In previous studies, we have shown that TCI induces strong systemic and mucosal immune responses (7, 11, 12, 15, 17, 32). However, it was not clear from previous studies whether LCs migrate beyond the direct DLNs after topical application of LT or whether they migrate to distal sites to stimulate T- and B-cell responses (8, 17). To determine whether LT-activated LCs would migrate primarily to the DLNs, FITC in PBS admixed with LT was placed on the skin at the dorsal caudal region, and the DLNs and non-DLNs were harvested at 24 h. Only cells isolated from inguinal lymph nodes contained a FITC+ population (Fig. 1J), whereas axillary and cervical lymph nodes demonstrated near-background numbers of FITC+ cells (Fig. 1K and L). In similar studies using FITC in organic solvent without LT, or with fluorescent-labeled protein OVA or LT, migration only to the specific draining nodes was seen after topical applications to the skin surface of the head, thorax, and abdomen, respectively (data not shown). These results suggest that the vast majority of adjuvant-activated LCs migrate only to the DLN proximal to the site of application, making the DLN a desirable target for an immunostimulation strategy.
The LT patch augments antibody responses to injected influenza virus vaccine.
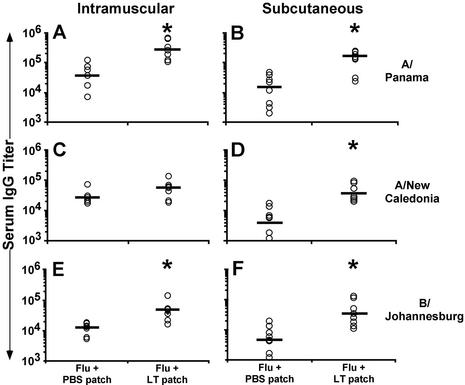
Based on our finding that LT increases DC migration to DLNs, we hypothesized that topical application of LT could enhance immune responses to antigen administered by systemic routes if applied in a way that both injected antigen and topically applied LT targeted the same DLNs. To test the hypothesis that topically applied LT potentiates the immune response to injected influenza virus vaccine, mice were immunized by routes used in human immunizations (s.c. or i.m.) with influenza virus vaccine, and a patch containing PBS or LT was applied on the dorsal caudal region. While i.m. or s.c. injections induced similar levels of serum IgG to all three influenza virus strains, the responses were significantly increased by LT-IS patch application (all three strains in the s.c. group; P < 0.05) (Fig. 2B, D, and F) and in the A/Panama and B/Johannesburg strains in the i.m. group (Fig. 2A and E).
FIG. 2.
An LT-IS patch enhances the immune response to i.m. and s.c. injected antigen. Mice were immunized on days 0 and 14 by i.m. or s.c. injection with 5 or 1.5 μg of trivalent influenza virus vaccine, respectively. LT (50 μg) or PBS (no LT) was loaded onto a patch applied to the skin for 1 h. Serum collected 2 weeks after the second immunization was measured by ELISA for IgG titers to A/Panama (panels A and B), A/New Caledonia (panels C and D), and B/Johannesburg (panels E and F). Individual titers are displayed (open circles) with the corresponding geometric mean (bars) for each group. Groups marked with an asterisk indicate a significant (P ≤ 0.05) increase from their respective group receiving a PBS patch.
To further confirm the importance of targeting antigen and adjuvant to the DLN, mice were injected with trivalent influenza virus vaccine by s.c., i.m., and i.d. routes, and patches were placed on the dorsal caudal surface (Fig. 3). i.d. injection of vaccine was done directly at the site of the LT-IS patch placement, versus the base of the tail (s.c.) or lateral thigh (i.m), to determine whether LT delivered within closer anatomical proximity to the patch would be more efficient at stimulating the responses. The results showed that responses to all three strains were significantly increased by the LT-IS patch with all three immunization routes. The LT-IS patch was especially efficient when influenza virus vaccine was administered i.d. at the site of placement (Fig. 3A, D, and G). The increased responses to the i.d. injection were LT dose dependent, and patches containing as little as 5 μg of LT were sufficient to augment specific antibody titers. Significant increases in antibody titers were achieved by s.c. injection with LT-IS patches containing 25 and 50 μg of LT (Fig. 3B, E, and H) and by i.m. injection with 50 μg of LT (Fig. 3C, F, and I). The three routes of immunization without the LT-IS patch induced comparable influenza virus-specific antibody responses independent of route. These studies suggested that antigen and adjuvant should be delivered in close anatomical proximity to obtain maximum efficiency with the LT-IS patch application, consistent with the need to target the same DLNs with the patch and injection.
FIG. 3.
An LT-IS patch enhances the immune response to antigen delivered by i.d., i.m., or s.c. injection. Mice were immunized on days 0 and 14 by i.d., i.m., or s.c. injection of 5 μg of influenza virus vaccine and application of an adhesive patch containing 5, 25, or 50 μg of LT or PBS alone overnight over the injection site. Serum was collected and analyzed, and results were reported as described in the legend for Fig. 2.
The immunostimulant effect depends on targeting adjuvant and antigen to the same DLN.
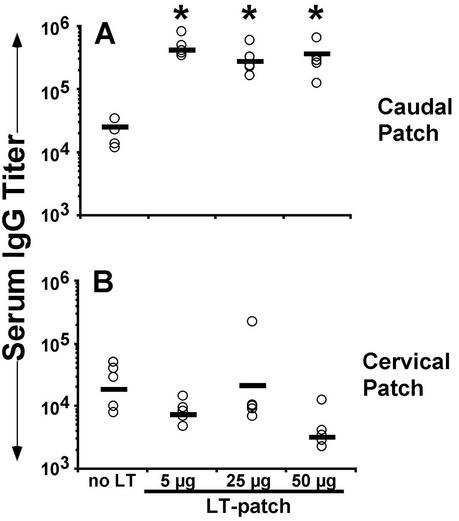
LT-IS patches placed directly over the injection site (i.d. and s.c.) or close to the injection site (i.m.) have been shown to augment immune responses to injected influenza virus vaccine (Fig. 3). To determine the effect of an LT-IS patch placed at a different site than the injection site, mice were immunized i.d. with influenza virus vaccine at the dorsal caudal region. Patches containing 0, 5, 25, or 50 μg of LT were placed at the same site or at the dorsal cervical region. As expected, mice that received an LT patch at the site of vaccine injection (caudal patch) had significantly higher antibody titers than the group with no LT (Fig. 4A). In contrast, when patches were placed in the cervical region draining to the cervical lymph nodes and injection was done in the dorsal caudal skin draining to the inguinal lymph nodes, the antibody levels were the same as the control group (Fig. 4B), while the serum antibody response to LT was strong (75,190 versus 104,275; P = 0.52 in the 50-μg group), indicating that the LT was adequately delivered in both groups. Similar results were seen in experiments done with i.m. injection (data not shown). This result confirms the need to target the same DLN with an IS patch in order to augment responses to an injected vaccine.
FIG. 4.
An LT-IS patch at a distal location does not enhance responses to injected influenza virus vaccine. Mice were immunized on days 0 and 14 by i.d injection with 5 μg of trivalent influenza virus vaccine at the dorsal caudal region, and LT (5, 25, or 50 μg) or PBS (no LT) was loaded onto a patch and applied on the skin over the site of injection (A) or at the dorsal cervical region (B). Serum collected 2 weeks after the second immunization was measured by ELISA for B/Johannesburg-specific IgG titers. Individual titers are displayed (open circles) with the corresponding geometric mean (bar) for each group.
Mucosal antibodies to influenza virus.
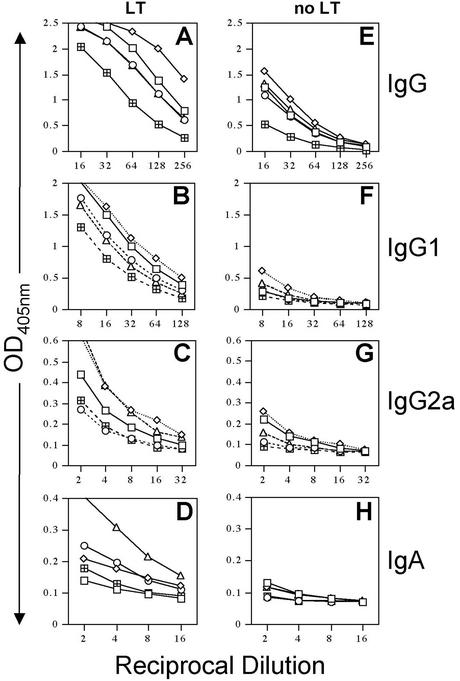
Although serum antibody titers correlate with protection against human influenza virus, mucosal antibody responses may also contribute to protection against influenza virus infection (14). To determine whether an LT-IS patch would stimulate mucosal antibody responses, lung lavage fluid was assayed for the presence of influenza virus A/Panama antibodies (Fig. 5). As expected, lung IgG responses (total IgG and IgG1, and IgG2a subclasses) to influenza virus A/Panama were significantly increased in mice immunized with influenza virus injection and the LT-IS patch compared to mice immunized by injection alone (Fig. 5A, B, and C versus E, F, and G). A/Panama-specific IgA responses appeared to be increased by the addition of an LT-IS patch, compared to placebo groups (Fig. 5D and H). This is consistent with the previous observation of the induction of antigen-specific mucosal secretory IgA in the context of TCI (35).
FIG. 5.
An LT-IS patch enhances mucosal antibody responses to influenza virus in the lung. Mice (as described in the legend for Fig. 3) were immunized by i.d. injection of influenza virus vaccine and received either a PBS or a 50-μg LT patch. Lung washes were collected 3 weeks after the third immunization and analyzed for IgG (A and E), IgG1 (B and F), IgG2a (C and G), and IgA (D and H) specific for A/Panama.
LT patch augments HAI titers.
Neutralizing antibodies against influenza virus are the standard marker of protective immunity against influenza virus infection (5, 19). To ensure that an LT-IS patch induces functional antibodies, HAI titers were measured. As shown in Table 1, the HAI titers were markedly enhanced with the use of an LT-IS patch, and the increase in titers was LT dose dependent. Although the magnitude of the responses was lower, enhancement of HAI titers was also seen with groups that were injected i.m. or s.c. with the addition of an LT-IS patch (data not shown).
TABLE 1.
HAI titers to influenza A and B virus strains
| Influenza virus strain | LT (μg) | HAI titer in sample no.:
|
Mean HAI titera | % Positiveb | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| A/New Caledonia | 0 | 20 | 5 | 20 | 5 | 5 | 11 | 0 |
| 5 | 40 | 80 | 80 | 40 | 40 | 56 | 100 | |
| 25 | 80 | 40 | 40 | 80 | 40 | 56 | 100 | |
| 50 | 320 | 80 | 160 | 160 | 80 | 160 | 100 | |
| A/Panama | 0 | 5 | 5 | 5 | 5 | 5 | 5 | 0 |
| 5 | 80 | 40 | 20 | 20 | 10 | 34 | 40 | |
| 25 | 10 | 40 | 20 | 20 | 20 | 22 | 20 | |
| 50 | 320 | 10 | 80 | 80 | 20 | 102 | 60 | |
| B/Guangdong | 0 | 80 | 5 | 10 | 5 | 10 | 22 | 20 |
| 5 | 40 | 160 | 160 | 80 | 320 | 152 | 100 | |
| 25 | 160 | 640 | 160 | 160 | 80 | 240 | 100 | |
| 50 | 2560 | 40 | 640 | 160 | 80 | 696 | 100 | |
Indicates the arithmetic mean of five individual titers 3 weeks after the third immunization.
The percentage of samples for each group with HAI titers of ≥40. Preimmune samples had HAI titers of ≤5 for all three viral strains.
Enhanced T-cell responses in a disease target tissue.
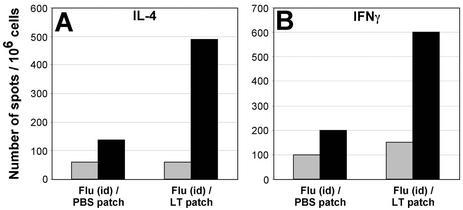
Previous studies have shown that TCI elicits systemic T-cell responses in sites beyond the DLN (spleen, distal nodes) (17, 18, 35). The effect of application of an LT-IS patch upon the generation of influenza virus antigen-specific T cells was evaluated using single-cell suspensions from perfused whole lung prepared from groups of mice immunized with and without the LT-IS patch. Antigen-induced IL-4 and IFN-γ were detected using an ELISPOT method. The application of an LT-IS patch significantly increased the number of influenza virus A-specific T cells producing both IFN-γ and IL-4 in the lungs (Fig. 6A and B, respectively). This is consistent with the enhancement of both mucosal IgG1 and IgG2a found in lung washes (Fig. 5B, C, F, and G) and suggests that both Th1 and Th2 T-cell responses are up-regulated with an LT-IS patch.
FIG. 6.
Influenza virus-specific lung T cells from skin-immunized mice secrete IFN-γ and IL-4. Mice were immunized as described in the legend for Fig. 4. Three weeks after the third immunization, lung cells were isolated and cultured in the presence of influenza virus A/Panama split virus (black columns) or medium alone (gray columns). Spots indicating the presence of IL-4- (A) or IFN-γ- (B) secreting cells were counted using a dissecting microscope.
Adjuvant and antigen loading of APCs.
To visualize the migration and localization of an injected antigen and topically applied LT, we applied AlexaFluor 488 conjugated to LT (AF-LT) and AlexaFluor 633 conjugated to the model antigen OVA (AF-OVA). Although these studies used OVA for purposes of antigen tracking, experiments performed to determine the LT-IS patch effect have shown that both cellular and humoral responses are also enhanced by the application of an LT-IS patch in mice parenterally (i.m. or i.d.) immunized with OVA (data not shown). For antigen-adjuvant tracking studies, labeled antigen was injected and labeled adjuvant was placed topically, and DLN were removed after 24 h to determine whether injected antigen and topical adjuvant were taken up by separate or the same APCs. These studies confirmed that injected antigen and topical adjuvant could be found only in the specific DLN (data not shown). Three sets of labeled populations can be distinguished on the dot plots (Fig. 7): AF-OVA single-positive cells (upper left quadrant), AF-LT single-positive cells (lower right), and AF-OVA+ AF-LT+ double-positive cells (upper right). The double-positive populations (adjuvant plus antigen) were seen in the i.d., s.c., and TCI groups (Fig. 7A, B, and D, upper right). These studies suggested that the adjuvant directly targeted at least a population of antigen-laden APCs. By contrast, in animals given OVA i.m. (lateral thigh), only AF-OVA+ or AF-LT+ cells were detected (Fig. 7C); no double-positive cells were seen.
The absence of double-positive cells in the i.m. groups suggests that LT-activated APCs can exert their immune-enhancing effects by bystander means, such as cytokine secretion, as well as by direct effects on the APC, as suggested by double-positive cells in the i.d., s.c., and TCI groups. Although antibody results indicated that the i.m. route requires a higher dose of LT in the IS patch to enhance the influenza virus-specific responses, this may reflect the inefficiency of a bystander effect or may result from the fact that antigen injected i.m. in the lateral thigh tracks to both the inguinal and popliteal LN, making the amount of antigen available in the inguinal node after i.m. injection lower compared to that after i.d. or s.c. injection.
To further explore the possibility that the effects on OVA-laden APCs in the i.m. groups were indirect, the OVA+ cells from groups of mice that received an LT-IS patch or placebo patches were tested for the expression of the LC activation marker CD86. Following i.d. and s.c. injection, OVA+ cells showed an up-regulated CD86 phenotype when isolated from mice with an LT-IS patch compared to placebo patch groups (Fig. 7E and F), implying a direct effect on the APCs by LT. This was also the case for cells isolated after TCI administration of both OVA and LT (Fig. 7H), which would be expected to load APCs directly with both adjuvant and antigen. For i.m. injection, the expression of CD86 was the same on OVA+ cells, with or without the use of an LT-IS patch (Fig. 7G). Thus, i.d. and s.c. injections resulted in LT-activated, antigen-laden APCs similar to topically applied adjuvant and antigen, whereas in the i.m. group OVA-laden APCs were not activated. These data suggest that adjuvant exerts both direct and indirect enhancing effects on antigen-loaded APCs.
LT-IS patch and antigen processing.
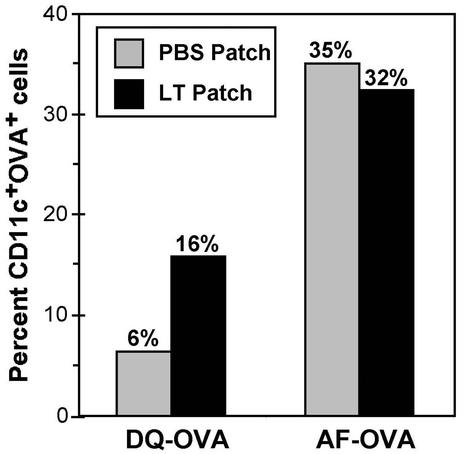
As the LT-IS patch appeared to augment both B- and T-cell-mediated responses, we reasoned that LT might affect antigen processing. To assess the role of an LT-IS patch on APCs, we used OVA coupled with a quenched fluorophore (DQ-OVA) (4) to monitor antigen processing, as DQ-OVA emits fluorescence only if proteolytic digestion occurs in the endosome in the first step of antigen processing by APCs. An unquenched fluorescent antigen (AF-OVA) that can elicit fluorescence without proteolysis was used as control. With DQ-OVA, a two- to threefold increase in fluorescence could be detected in groups with LT patches compared to the group with the PBS patch (16% versus 6% CD11c+ OVA+ cells) (Fig. 8). As expected, there was no clear difference in fluorescence for the groups treated with AF-OVA with and without LT (35% versus 32% CD11c+ OVA+ cells), as AF-OVA does not require proteolysis to fluoresce. This result suggests that LT-activated LCs demonstrate increased antigen processing in response to the LT-IS patch, consistent with the concept of greater antigen presentation and T-cell priming.
FIG. 8.
Application of an IS patch increases the amount of antigen processed by CD11c+ dendritic cells. C57BL/6 mice (n = 4) were injected i.d. in the dorsal caudal region with 300 μg of DQ-OVA or AlexaFluor 488-conjugated OVA (AF-OVA). A PBS (grey columns) or LT (black columns) patch was applied onto the pretreated dorsal caudal surface directly on top of the injection site. The mice were euthanized 20 h after immunization, and their inguinal (draining) LN were excised, separated into single-cell suspensions, and stained with fluorescently labeled antibodies. The bars represent the percentage of large, granular, CD11c+, CD19−, live inguinal LN cells containing fluorescent OVA.
DISCUSSION
This study shows that an adjuvant such as LT administered as an LT-IS patch can significantly increase immune responses to influenza virus vaccine administered by injection. The effect of LT was manifested by higher serum antibodies and HAI titers and enhanced levels of mucosal influenza virus-specific IgA and IgG. Increased numbers of antigen-specific IFN-γ- and IL-4-producing T cells were also found in the lungs of mice parenterally immunized with influenza virus vaccine and who received an LT-IS patch at the time of injection. Finally, the effect of topical LT on increased numbers and on the activation state of migrating CD11c+ cells into the DLN appears to be tied to enhanced presentation of antigen delivered to the same DLN. These data together suggest that LT activates skin APCs, which then enhance the immune response to antigens injected over the same DLN.
The LT-IS patch strategy takes advantage of the potent immunostimulating properties of LT while avoiding the systemic side effects seen with the use of adjuvants by mucosal or parenteral routes (9, 26, 29, 31). Topical immunization appears to target the most superficial layer of the skin, the epidermis, which contains a significant number of LCs, which are known to have an important role in immune surveillance. Stimulating agents, such as microbes, induce activation of LCs and migration from the skin to the DLNs (21, 23, 34). Cell-tracking studies have confirmed that antigens applied on the skin are taken to the DLNs by LCs (22, 23). These LCs display an activated phenotype and up-regulated MHC class II molecules (20, 22). Our data consistently show that the adjuvant LT increases the migration of DCs from the skin to the DLN and up-regulates their activation state. In studies using TCI, robust T-cell and antibody responses to the vaccines are seen only when adjuvant is used, suggesting that the adjuvant effect on APCs results in the immune enhancement. Taken together, the data in this study and previous studies suggest that the adjuvant exerts its effect through the LC, although other skin APCs may be engaged.
While the effect of an LT-IS patch was evident for all routes of immunization (i.d., s.c., and i.m.), the precise mechanism for immune augmentation will require further investigation. In the context of topical delivery of adjuvant and antigen (TCI), the APC appears to be loaded with both adjuvant and antigen, as might be expected. We had hypothesized that the LT-IS patch strategy might exert its enhancement through bystander effects, such as cytokine secretion in the microenvironment of the DLN affecting and enhancing the antigen presentation of the antigen-loaded APC. The tracking studies suggested that in scenarios where the antigen and adjuvant were delivered in similar anatomical spaces (i.d., s.c., TCI), the antigen and adjuvant were loaded in the same APC (Fig. 7). However, in the studies using i.m. injection, immune enhancement was seen even though the APCs appeared to be separately loaded. Thus, while immune enhancement by an LT-IS patch may be due to direct effects of the adjuvant on the APC, indirect bystander effects mediated by cytokines or chemokines secreted in the microenvironment of the DLN may also play a role (2).
Vaccines with low immunogenicity in particular require the use of a strong adjuvant to enhance the antigen-presenting environment by increasing the expression of inflammatory cytokines and APC costimulatory molecules (2, 27). Inflammatory stimuli induce a rapid and transient boost of MHC class II synthesis and increase the half-life of MHC class II molecules, resulting in large numbers of long-lived peptide-loaded MHC class II molecules capable of stimulating T cells even after several days (3). Similarly, bacteria can increase MHC class I molecules' stabilization by threefold of their half-life, leading to efficient presentation in the DLNs (30). In agreement with these findings, our data suggest that an LT-IS patch can increase antigen processing in LCs, increase levels of costimulating molecules (CD86), and thereby augment the effect or immune responses important to protective immunity.
The present data clearly show that an LT-IS patch elicits qualitative and quantitative enhancement of antigen-specific responses. We have found that the LT-IS patch enhances the immune response to a variety of antigens (data not shown), suggesting that this strategy may be used in several contexts, such as enhancing immune responses in elderly vaccinees to influenza virus and pneumococcal vaccines, in established cancer immunotherapy regimens that lack sufficient immune stimulation, or in dose sparing in contexts where vaccine supply is critical (pandemic influenza). When immune responses to appropriate strains of influenza virus are elicited through vaccination, the vaccine is highly effective in prevention of influenza-related morbidity and mortality (25). However, the elderly immune response rates to influenza virus vaccination are unsatisfactory and clearly much lower compared to healthy adults (6). Although the use of adjuvants has been shown to enhance influenza virus responses in healthy adults (31) and in the elderly (9), the use of an LT-IS patch may both enhance the influenza virus-specific immune response and improve the tolerability of adjuvant use. Clearly, the biology of an IS strategy needs to be confirmed in the context of target settings such as the elderly, immune suppressed, and healthy human populations. However, the LT-IS patch appears to be a practical strategy, and LT effects are well established in human studies (12, 15). Clinical studies will confirm whether an LT-IS patch can augment the immune response to injected vaccines and thus provide an additional important tool for immunization strategies.
Acknowledgments
We thank Vernisha Simmons for technical assistance and Wanda Hardy for preparing the manuscript.
REFERENCES
- 1.Arrington, J., R. P. Braun, L. Dong, D. H. Fuller, M. D. Macklin, S. W. Umlauf, S. J. Wagner, M. S. Wu, L. G. Payne, and J. R. Haynes. 2002. Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines. J. Virol. 76:4536-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Cella, M., A. Engering, V. Pinet, J. Pieters, and A. Lanzavecchia. 1997. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388:782-787. [DOI] [PubMed] [Google Scholar]
- 4.Daro, E., B. Pulendran, K. Brasel, M. Teepe, D. Pettit, D. H. Lynch, D. Vremec, L. Robb, K. Shortman, H. J. McKenna, C. R. Maliszewski, and E. Maraskovsky. 2000. Polyethylene glycol-modified GM-CSF expands CD11bhighCD11chigh but not CD11blowCD11chigh murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 165:49-58. [DOI] [PubMed] [Google Scholar]
- 5.Davies, J. R., and E. A. Grilli. 1989. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol. Infect. 102:325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bruijn, I. A., E. J. Remarque, C. M. Jol-van der Zijde, M. J. van Tol, R. G. Westendorp, and D. L. Knook. 1999. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J. Infect. Dis. 179:31-36. [DOI] [PubMed] [Google Scholar]
- 7.El-Ghorr, A. A., R. M. Williams, C. Heap, and M. Norval. 2000. Transcutaneous immunisation with herpes simplex virus stimulates immunity in mice. FEMS Immunol. Med. Microbiol. 29:255-261. [DOI] [PubMed] [Google Scholar]
- 8.Enioutina, E. Y., D. Visic, and R. A. Daynes. 2000. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine 18:2753-2767. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini, R., T. Pozzi, E. Montomoli, E. Fragapane, F. Senatore, M. Minutello, and A. Podda. 2001. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur. J. Epidemiol. 17:135-140. [DOI] [PubMed] [Google Scholar]
- 10.Glenn, G. M., D. Lang, and D. Taylor. 2000. Transcutaneous immunization, p. 91-93. In M. A. Gerber (ed.), The Jordan report 2000: accelerated development of vaccines. Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md.
- 11.Glenn, G. M., T. Scharton-Kersten, R. Vassell, C. P. Mallett, T. L. Hale, and C. R. Alving. 1998. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 161:3211-3214. [PubMed] [Google Scholar]
- 12.Glenn, G. M., D. N. Taylor, X. Li, S. Frankel, A. Montemarano, and C. R. Alving. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 6:1403-1406. [DOI] [PubMed] [Google Scholar]
- 13.Graham, B. S., M. C. Keefer, M. J. McElrath, G. J. Gorse, D. H. Schwartz, K. Weinhold, T. J. Matthews, J. R. Esterlitz, F. Sinangil, P. E. Fast, et al. 1996. Safety and immunogenicity of a candidate HIV-1 vaccine in healthy adults: recombinant glycoprotein (rgp) 120. A randomized, double-blind trial. Ann. Intern. Med. 125:270-279. [DOI] [PubMed] [Google Scholar]
- 14.Greenbaum, E., A. Furst, A. Kiderman, B. Stewart, R. Levy, M. Schlesinger, A. Morag, and Z. Zakay-Rones. 2001. Serum and mucosal immunologic responses in children following the administration of a new inactivated intranasal anti-influenza vaccine. J. Med. Virol. 65:178-184. [PubMed] [Google Scholar]
- 15.Guerena-Burgueno, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halperin, S. A., B. Smith, T. Mabrouk, M. Germain, P. Trepanier, T. Hassell, J. Treanor, R. Gauthier, and E. L. Mills. 2002. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 20:1240-1247. [DOI] [PubMed] [Google Scholar]
- 17.Hammond, S. A., M. Guebre-Xabier, J. Yu, and G. M. Glenn. 2001. Transcutaneous immunization: an emerging route of immunization and potent immunostimulation strategy. Crit. Rev. Ther. Drug Carrier Sys. 18:503-526. [PubMed] [Google Scholar]
- 18.Hammond, S. A., D. Walwender, C. R. Alving, and G. M. Glenn. 2001. Transcutaneous immunization: T-cell responses and boosting of existing immunity. Vaccine 19:2701-2707. [DOI] [PubMed] [Google Scholar]
- 19.Hirota, Y., M. Kaji, S. Ide, J. Kajiwara, K. Kataoka, S. Goto, and T. Oka. 1997. Antibody efficacy as a keen index to evaluate influenza vaccine effectiveness. Vaccine 15:962-967. [DOI] [PubMed] [Google Scholar]
- 20.Jakob, T., and M. C. Udey. 1999. Epidermal Langerhans cells: from neurons to nature's adjuvants. Adv. Dermatol. 14:209-258. [PubMed] [Google Scholar]
- 21.Johnston, L. J., G. M. Halliday, and N. J. King. 2000. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J. Investig. Dermatol. 114:560-568. [DOI] [PubMed] [Google Scholar]
- 22.Kripke, M. L., C. G. Munn, A. Jeevan, J. M. Tang, and C. Bucana. 1990. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J. Immunol. 145:2833-2838. [PubMed] [Google Scholar]
- 23.Macatonia, S. E., S. C. Knight, A. J. Edwards, S. Griffiths, and P. Fryer. 1987. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J. Exp. Med. 166:1654-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, J. T. 1997. Development of an adjuvant to enhance the immune response to influenza vaccine in the elderly. Biologicals 25:209-213. [DOI] [PubMed] [Google Scholar]
- 25.Martinez, F. D. 2001. The coming-of-age of the hygiene hypothesis. Respir. Res. 2:129-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 27.Morein, B., K. Lovgren-Bengtsson, and J. Cox. 1996. Modern adjuvants, functional aspects, p. 243-263. In S. H. E. Kaufmann (ed.), Concepts in vaccine development. Walter de Gruyter, Berlin, Germany.
- 28.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 29.Pittman, P. R., G. Kim-Ahn, D. Y. Pifat, K. Coonan, P. Gibbs, S. Little, J. G. Pace-Templeton, R. Myers, G. W. Parker, and A. M. Friedlander. 2002. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine 20:1412-1420. [DOI] [PubMed] [Google Scholar]
- 30.Rescigno, M., S. Citterio, C. Thery, M. Rittig, D. Medaglini, G. Pozzi, S. Amigorena, and P. Ricciardi-Castagnoli. 1998. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl. Acad. Sci. USA 95:5229-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salk, J. E., M. Contakos, A. M. Laurent, M. Sorensen, A. J. Rapalski, H. Simmons, and H. Sandberg. 1953. Use of adjuvants in studies on influenza immunization. Degree of persistence of antibody in human subjects two years after vaccination. JAMA 151:1169-1175. [DOI] [PubMed] [Google Scholar]
- 32.Scharton-Kersten, T., J. Yu, R. Vassell, D. O'Hagan, C. R. Alving, and G. M. Glenn. 2000. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect. Immun. 68:5306-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh, M., and D. O'Hagan. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075-1081. [DOI] [PubMed] [Google Scholar]
- 34.Udey, M. C. 1997. Cadherins and Langerhans cell immunobiology. Clin. Exp. Immunol. 10(Suppl. 1):6-8. [PubMed] [Google Scholar]
- 35.Yu, J., F. Cassels, T. Scharton-Kersten, S. A. Hammond, A. Hartman, E. Angov, B. Corthesy, C. Alving, and G. Glenn. 2002. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect. Immun. 70:1056-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]