Abstract
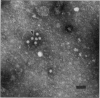
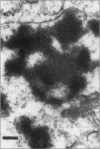
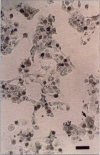
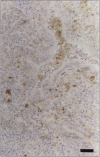
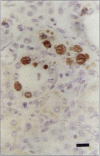
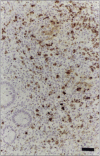
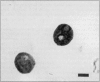
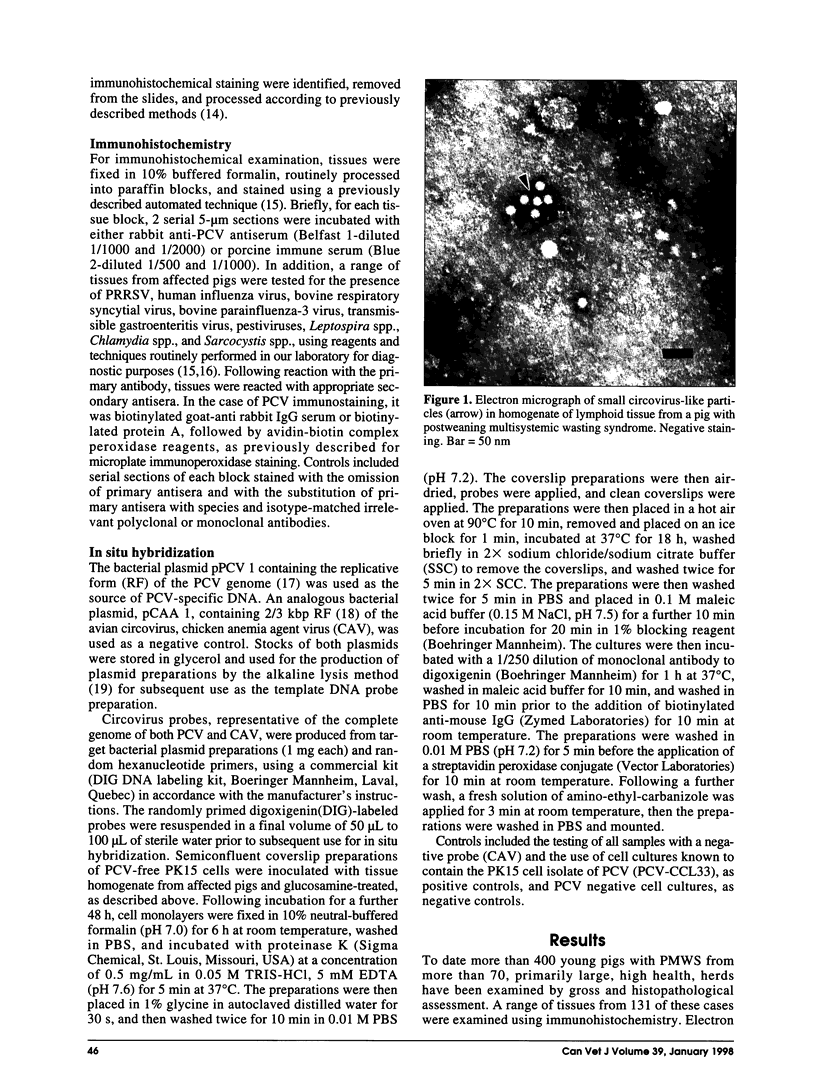
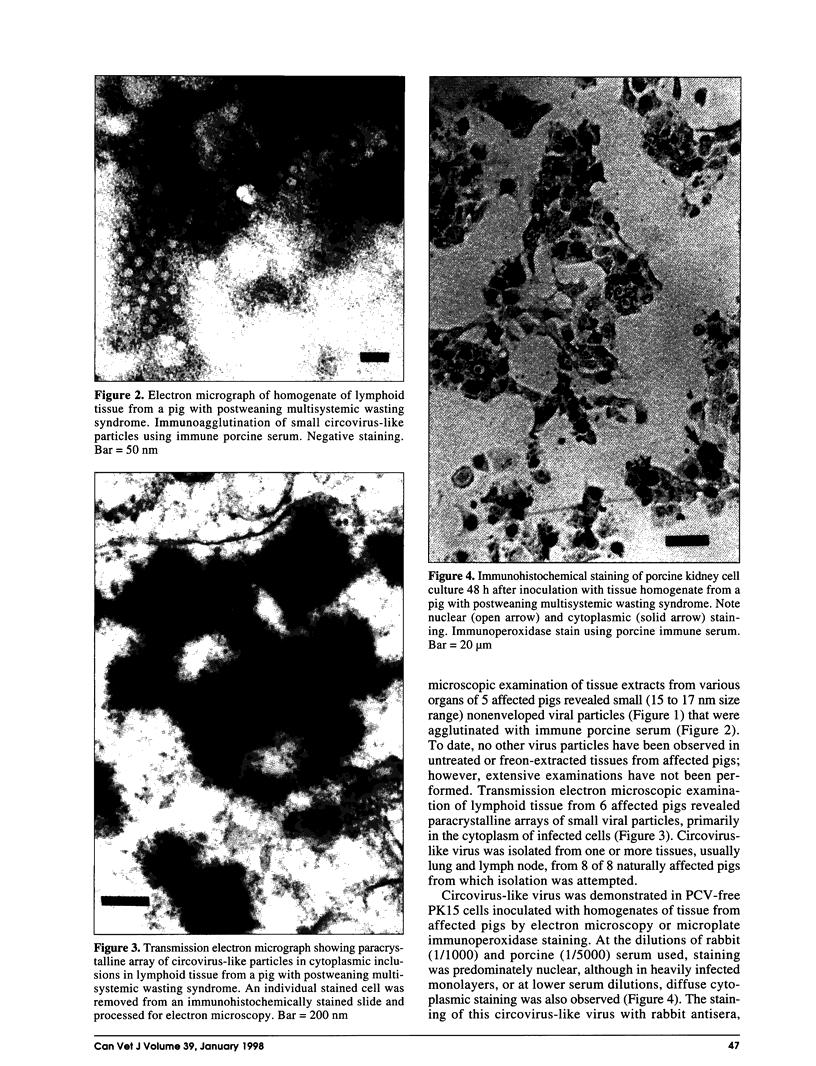
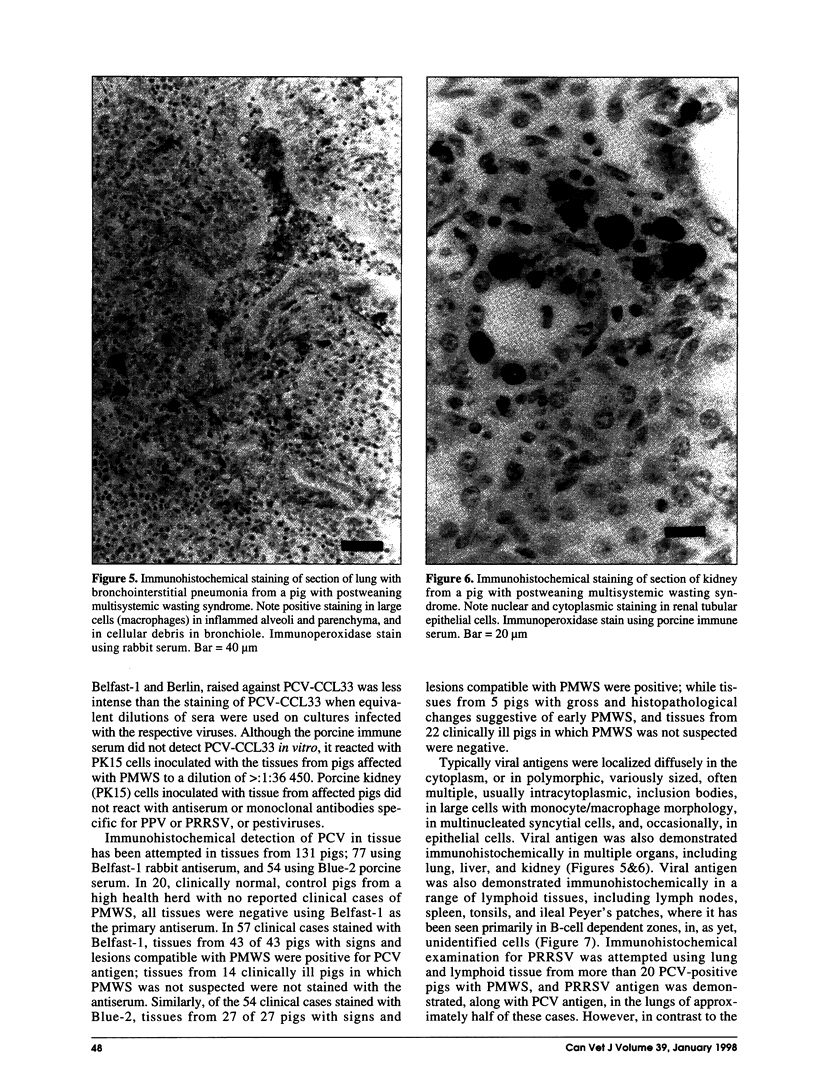
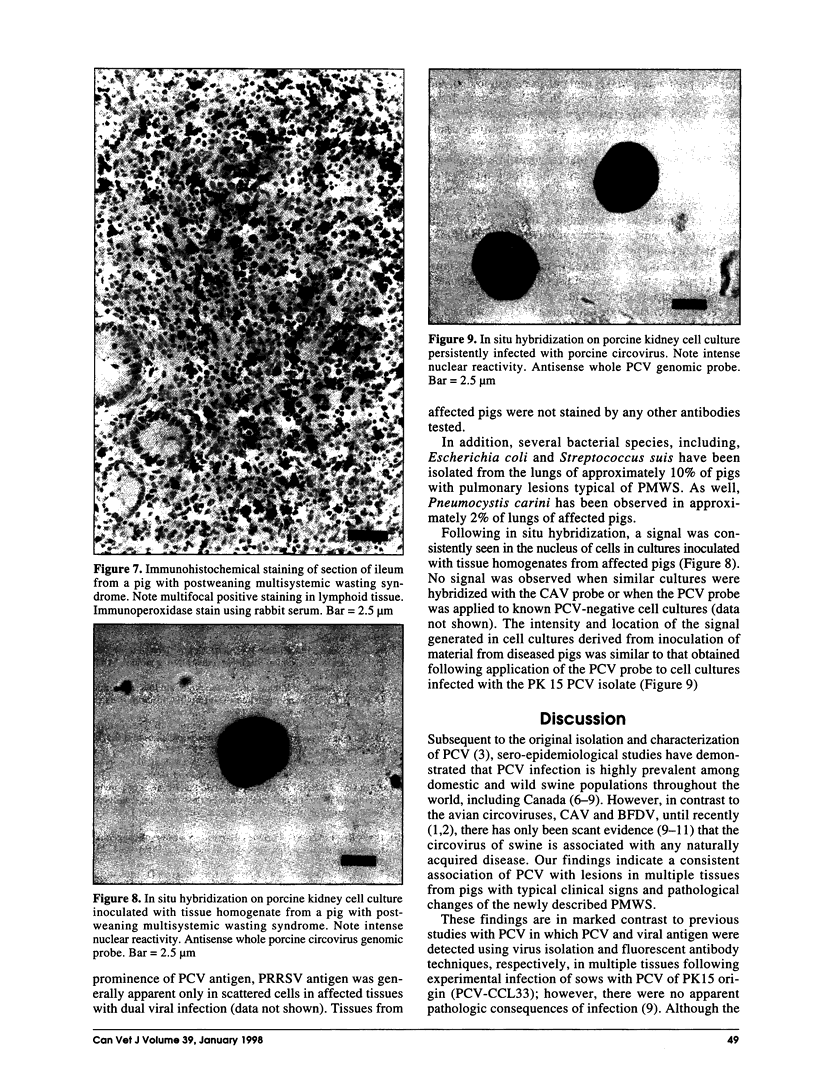
Postweaning multisystemic wasting syndrome (PMWS), an apparently new disease, has been recognized in swine herds in western Canada. Young pigs with this disease have progressive weight loss, tachypnea, dyspnea, and jaundice, accompanied by interstitial pneumonia, lymphadenopathy, hepatitis, and nephritis. We examined more than 400 pigs from more than 70 herds in Alberta, Saskatchewan, and Manitoba with cases of PMWS. A small virus was isolated from a range of tissues from 8 of 8 affected pigs examined. The agent was identified as a circovirus-like virus using electron microscopy, immunohistochemical staining with porcine and rabbit immune serum, and in situ hybridization. Immunohistochemical examination of tissues from more than 100 affected pigs has revealed widespread viral antigen, often contained in circovirus-like inclusion bodies, in lesions from numerous organs. Although Koch's postulates remain to be fulfilled, these results demonstrate a high degree of association between the presence of the circovirus-like virus and PMWS in affected swine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair B. M., McNeilly F., McConnell C. D., Todd D., Nelson R. T., McNulty M. S. Effects of chicken anemia agent on lymphokine production and lymphocyte transformation in experimentally infected chickens. Avian Dis. 1991 Oct-Dec;35(4):783–792. [PubMed] [Google Scholar]
- Allan G. M., Mackie D. P., McNair J., Adair B. M., McNulty M. S. Production, preliminary characterisation and applications of monoclonal antibodies to porcine circovirus. Vet Immunol Immunopathol. 1994 Nov;43(4):357–371. doi: 10.1016/0165-2427(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Allan G. M., McNeilly F., Cassidy J. P., Reilly G. A., Adair B., Ellis W. A., McNulty M. S. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet Microbiol. 1995 Apr;44(1):49–64. doi: 10.1016/0378-1135(94)00136-k. [DOI] [PubMed] [Google Scholar]
- Allan G. M., McNeilly F., Foster J. C., Adair B. M. Infection of leucocyte cell cultures derived from different species with pig circovirus. Vet Microbiol. 1994 Aug 1;41(3):267–279. doi: 10.1016/0378-1135(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Blank H., Davis C., Collins C. Electron microscopy for the diagnosis of cutaneous viral infections. Br J Dermatol. 1970;83(Suppl):69–80. doi: 10.1111/j.1365-2133.1970.tb12866.x. [DOI] [PubMed] [Google Scholar]
- Chang S. F., Sgro J. Y., Parrish C. R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J Virol. 1992 Dec;66(12):6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac G. C., Afshar A. Porcine circovirus antigens in PK-15 cell line (ATCC CCL-33) and evidence of antibodies to circovirus in Canadian pigs. Can J Vet Res. 1989 Oct;53(4):431–433. [PMC free article] [PubMed] [Google Scholar]
- Haines D. M., Chelack B. J. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Invest. 1991 Jan;3(1):101–112. doi: 10.1177/104063879100300128. [DOI] [PubMed] [Google Scholar]
- Haines D. M., Clark E. G. Enzyme immunohistochemical staining of formalin-fixed tissues for diagnosis in veterinary pathology. Can Vet J. 1991 May;32(5):295–302. [PMC free article] [PubMed] [Google Scholar]
- Latimer K. S., Rakich P. M., Kircher I. M., Ritchie B. W., Niagro F. D., Steffens W. L., 3rd, Lukert P. D. Extracutaneous viral inclusions in psittacine beak and feather disease. J Vet Diagn Invest. 1990 Jul;2(3):204–207. doi: 10.1177/104063879000200309. [DOI] [PubMed] [Google Scholar]
- Meehan B. M., Creelan J. L., McNulty M. S., Todd D. Sequence of porcine circovirus DNA: affinities with plant circoviruses. J Gen Virol. 1997 Jan;78(Pt 1):221–227. doi: 10.1099/0022-1317-78-1-221. [DOI] [PubMed] [Google Scholar]
- Tischer I., Bode L., Peters D., Pociuli S., Germann B. Distribution of antibodies to porcine circovirus in swine populations of different breeding farms. Arch Virol. 1995;140(4):737–743. doi: 10.1007/BF01309961. [DOI] [PubMed] [Google Scholar]
- Tischer I., Peters D., Rasch R., Pociuli S. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch Virol. 1987;96(1-2):39–57. doi: 10.1007/BF01310989. [DOI] [PubMed] [Google Scholar]
- Tischer I., Rasch R., Tochtermann G. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl Bakteriol Orig A. 1974 Feb;226(2):153–167. [PubMed] [Google Scholar]
- Todd D., Creelan J. L., McNulty M. S. Dot blot hybridization assay for chicken anemia agent using a cloned DNA probe. J Clin Microbiol. 1991 May;29(5):933–939. doi: 10.1128/jcm.29.5.933-939.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd D., Niagro F. D., Ritchie B. W., Curran W., Allan G. M., Lukert P. D., Latimer K. S., Steffens W. L., 3rd, McNulty M. S. Comparison of three animal viruses with circular single-stranded DNA genomes. Arch Virol. 1991;117(1-2):129–135. doi: 10.1007/BF01310498. [DOI] [PubMed] [Google Scholar]