Abstract
Proinflammatory cytokines, including IL-1β and tumor necrosis factor-α (TNF-α), promote cancer cell adhesion and liver metastases by up-regulating the expression of vascular cell adhesion molecule-1 (VCAM-1) on hepatic sinusoidal endothelium (HSE). In this study, hepatic metastasis after intrasplenically injected mouse B16 melanoma (B16M) cells was reduced 84–95% in mice with null mutations for either IL-1β or the IL-1β-converting enzyme (ICE, caspase-1) compared with wild-type mice. On day 12, 47% of wild-type mice were dead compared with 19% of either IL-1β or ICE-deficient mice. In vitro, conditioned medium from B16M cells (B16M-CM) induced the release of TNF-α and IL-1β from cultures of primary murine HSE. The effect of B16M-CM on HSE resulted in increased numbers of B16M cells adhering to HSE, which was completely abrogated by a specific inhibitor of ICE, anti-IL-18 or IL-18-binding protein. Exogenous IL-18 added to HSE also increased the number of adhering melanoma cells; however, this was not affected by IL-1 receptor blockade or TNF neutralization but rather by anti-VCAM-1. These results demonstrate a role for IL-1β and IL-18 in the development of hepatic metastases of B16M in vivo. In vitro, soluble products from B16M cells stimulate HSE to sequentially release TNF-α, IL-1β, and IL-18. The IL-18 cytokine increases expression of VCAM-1 and the adherence of melanoma cells.
The adhesion of circulating cancer cells to capillary endothelia is a critical step in the initiation of metastasis (1). Vascular cell adhesion molecule-1 (VCAM-1), up-regulated by proinflammatory cytokines, facilitates the binding of leukocytes to activated endothelial cells. The adhesive function of VCAM-1 is also used by cancer cells to enhance metastatic implantation and spread (2). For example, IL-1β and tumor necrosis factor-α (TNF-α) are known to potentiate the metastasis of very late antigen-4-expressing mouse B16 melanoma (B16M) cells in lung tissue by a mechanism which involves the up-regulation of VCAM-1 expression on endothelial cells (3, 4). We also have demonstrated that IL-1 significantly contributes to hepatic colonization of B16M cells both in normal and lipopolysaccharide-treated mice (5, 6). In addition, mannose receptor-mediated murine hepatic sinusoidal endothelium (HSE) activation involves endogenous IL-1-mediated expression of VCAM-1, leading to increased B16M cell adhesion and metastasis (7). IL-1β-activated HSE cells release very late antigen-4-stimulating factors that potentiate B16M cell adhesion to HSE (8). Thus, IL-1 can create a prometastatic microenvironment for certain intrasinusoidally arrested very late antigen-4-expressing cancer cells.
In the present study, the role of the IL-1β-converting enzyme (ICE, caspase-1) in B16M melanoma metastasis in mice was investigated. ICE cleaves the precursors of both IL-1β and IL-18 (9, 10), and hence, inhibitors of ICE or mice with a null mutation for ICE result in a deficiency in the release of biologically active IL-1β as well as biologically active IL-18. To differentiate between the role of either cytokine in ICE-deficient mice, we compared B16M metastasis after intrasplenic injection of B16M cells into mice deficient in ICE or IL-1β. The role of IL-18 in metastatic spread via activation of HSE is unknown, although IL-18 is a proinflammatory cytokine with several similarities to IL-1β (11), including up-regulation of adhesion molecules (12). However, IL-18 is different from IL-1 in that IL-18 plays an essential role as an interferon-γ (IFN-γ)-inducing factor (13–15). IL-18 is a product of macrophages and particularly the Kupffer cells (13), and therefore may play a role in hepatic metastasis by altering the microenvironment of the hepatic sinusoidal wall.
The conditioned medium from B16M cells (B16M-CM) contains spontaneously released, unknown soluble “factors” that, in turn, stimulate the production of IL-1β and TNF-α as well as the expression of VCAM-1 on HSE (16). Inhibitors of ICE, IL-1 receptor blockade, and neutralization of TNF were employed to characterize the cytokine cascade induced by these soluble factors on HSE activation. In addition, the role of IL-18 in B16M adhesion to HSE was investigated by using the newly described, naturally occurring IL-18 binding protein (IL-18BP) (17). Although IL-18BP circulates in healthy subjects and appears to be a natural inhibitor of production of the T-cell helper cytokine IFN-γ (17), there may be a role for IL-18BP in regulating host resistance to cancer by either affecting the adhesion of cancer cells to vascular endothelium or suppressing the T-cell helper function of immunosurveillance to malignant cells.
Materials and Methods
Reagents.
Rat anti-mouse IgG and rat anti-mouse VCAM-1 mAb were obtained from Serotec. Recombinant murine IL-1β was obtained from R & D Systems. Recombinant human IL-1 receptor antagonist (IL-1Ra) was a kind gift from Amgen Biologicals, and recombinant human TNF-binding protein (TNFbp), the native p55 TNF soluble receptor (18, 19) was a kind gift from Serono Laboratories (Randolph, MA). ICE inhibitor (ICEi) was purchased from Alexis Co. (San Diego, CA). Recombinant murine IL-18 and rabbit anti-mouse IL-18 IgG were purchased from PeproTech EC (London, U.K.). Recombinant human IL-18BP was produced as described (17). Reverse transcription–PCR for murine IL-18 was determined as described (20).
Culture of B16M Cells.
B16M cells intended for intrasplenic injection were cultured, maintained, and passaged as described (8). B16M-CM was prepared as follows: 5 × 105 B16M cells were plated in a 25-cm2 T-flask and cultured for 24 h in 5% FCS. Cells were cultured for an additional 24 h in serum-free medium (final cellular density of 6 × 104 cells/cm2). Supernatants were collected, diluted 1:3 in fresh serum-free medium, and passed through a 0.22 μm filter.
Quantitative B16M Cell Adhesion to Primary HSE Cultures.
HSE cells were separated from syngeneic mice, identified, and cultured as described (21). B16M cells were labeled with 2′,7′-bis-(2-carboxyethyl)-5,6-carboxyfluorescein-acetoxymethylester solution (Molecular Probes, Eugene, OR) as reported (6). HSE cells were incubated with various stimulants or inhibitors as indicated in each figure legend. Following the incubation times, the HSE was washed and the number of labeled cells determined. For the adherence cell assay, 2 × 105-labeled B16M cells were added to wells. After an 8-min incubation at 37°C, HSE cells were washed three times with fresh medium. The number of adhering cells was determined by using a quantitative method based on a previously described fluorescence measurement system (6). The percentage of B16M cells adhering to HSE was calculated as the relative value with respect to the initial number of added cells.
Cytokine Analysis.
Release of cytokines from cultures of primary HSE cells and B16M cells was measured by using specific ELISA kits for mouse IL-1β and TNF-α (R & D Systems). Circulating IFN-γ and IL-1β in mice was measured as described (22).
Hepatic Metastasis Assay.
The protocol was approved by the University of Colorado Health Sciences Animal Care Committee. IL-1β−/− and ICE−/− male mice were generated as described (23, 24). Six- to eight-week-old mice (housed five per cage) were used. Hepatic metastases were produced by the intrasplenic injection into anesthetized mice of 3 × 105 viable B16M melanoma cells suspended in 0.1 ml Hanks' balanced salt solution (6). Mice were killed under anesthesia on the 10th day after the injection of cancer cells. Liver tissue was fixed in PBS with 10% formaldehyde, pH 7.4, and processed for routine histology. Densitometric analysis of digitized microscopic images was used to discriminate metastatic B16M from normal hepatic tissue. The mean number of melanoma foci in 15 10 × 10 mm2 sections per liver was determined. The density of liver metastasis, which is the number of metastases per 100 mm3 of liver, was calculated with the stereological procedures described (5).
Results
Reduced Metastasis and Growth of B16M Cells Injected into IL-1β- and ICE-Deficient Mice.
Initial experiments examined mortality. Seven of 15 wild-type (WT) mice injected intrasplenically were dead on day 12, whereas only 3 out of 16 of both the IL-1β−/− and ICE−/− were dead on day 12. However, to quantitate the degree of hepatic metastasis ante mortem, the experiment was repeated and mice were killed on day 10 after the injection of tumor cells. Separated by 1 year, in two independent experiments and by using two different cultures of B16M cells, mice were injected intrasplenically into WT background, IL-1β−/− and ICE−/− mice. Ten days later, gross inspection demonstrated visible melanotic tumors in the spleen from all mice, without significant differences in size as evaluated by splenic weight. In contrast, a marked decrease in metastasis was present in the livers of IL-1β−/− and ICE−/− mice compared with WT mice (Fig. 1). Quantitative histological analyses on number and size of metastatic foci were performed to determine metastasis density (as number of foci per 100 mm3) and metastasis volume (percent organ occupancy). As shown in Table 1, compared with WT mice, hepatic metastasis density was significantly reduced (84–95%, P < 0.01) in the livers of IL-1β−/− and ICE−/− mice. These results indicate that most of the injected B16M cells were unable to implant in the hepatic tissue of the mutant mice. In addition, metastasis volume, an indicator of metastatic growth, was also significantly reduced in IL-1β−/− and ICE−/− mice six- to sevenfold (P < 0.01, Table 1). We also observed a difference in these metastasis parameters between IL-1β−/− and ICE−/− mice, particularly in experiment 1. The mean number of metastatic foci in the WT mice was 234; the IL-1β−/− mice, 25; and only 13 in the ICE−/− mice, which was a 95% reduction.
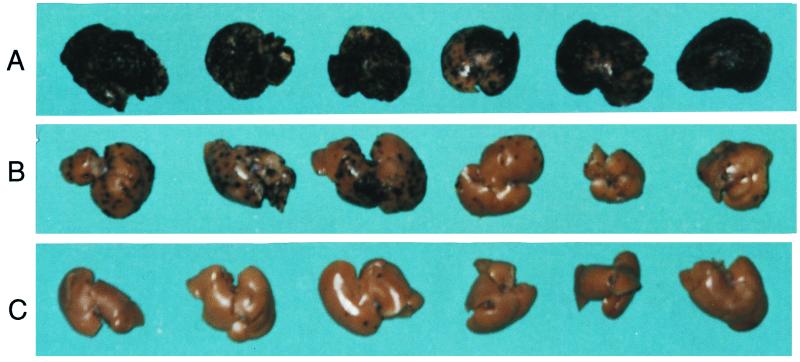
Figure 1.
Experimental hepatic colonization after intrasplenic injection of B16M cells into WT (A), IL-1β−/− (B), or ICE−/− (C) mice. Livers were removed on day 10 after injection of melanoma cells, fixed, and photographed. Metastases can be identified by black melanotic nodules. These livers are taken from mice depicted in Table 1, Experiment I.
Table 1.
Hepatic colonization following intrasplenically injected B16M cells into WT, IL-1β−/−, or ICE−/− mice
| Mouse group | Metastasis density, no. foci per 100 mm3 | Metastasis volume, % liver volume |
|---|---|---|
| Experiment I | ||
| WT | 234.16 ± 58.36* | 66.18% |
| IL-1β−/− | 25.18 ± 21.02† | 10.05% |
| ICE−/− | 13.56 ± 16.20† | 2.1% |
| Experiment II | ||
| WT | 198.40 ± 100.54 | 59.62% |
| IL-1β−/− | 33.79 ± 19.89† | 9.70% |
| ICE−/− | 27.73 ± 15.68† | 8.08% |
Means ± SD of two independent experiments performed one year apart (7–15 mice per group).
P < 0.01 with respect to WT mice by ANOVA and the Scheffe F test.
Autocrine IL-18 Mediates TNF-α- and IL-1β-Induced Adhesiveness on B16M-CM-Activated HSE.
Incubation of B16M-CM with HSE resulted in a significant (P < 0.01) increase in the adhesiveness of B16M cells as well as release of TNF-α and IL-1β into the HSE supernatant (Fig. 2). In the presence of ICEi, both the increased release in IL-1β as well as the increase in melanoma cell adhesiveness was completely abrogated, without decreasing TNF-α. The addition of anti-IL-18 IgG also completely abrogated the increase in adhesiveness but did not affect the release of IL-1β or TNF-α. There were no statistically significant changes in IL-1β or TNF-α levels or in the number of adhering B16M cells to HSE cells when these cells were treated with ICEi or anti-IL-18 IgG in the absence of B16M-CM (data not shown). These results suggest that soluble factors present in the B16M-CM induce a cascade that begins with the release of TNF-α and is followed sequentially by ICE-dependent release of IL-1β and IL-18. Because antimurine IL-18 completely reduced the increase in HSE adhesiveness, IL-18 appears to be responsible for the expression of VCAM-1 in activated HSE.
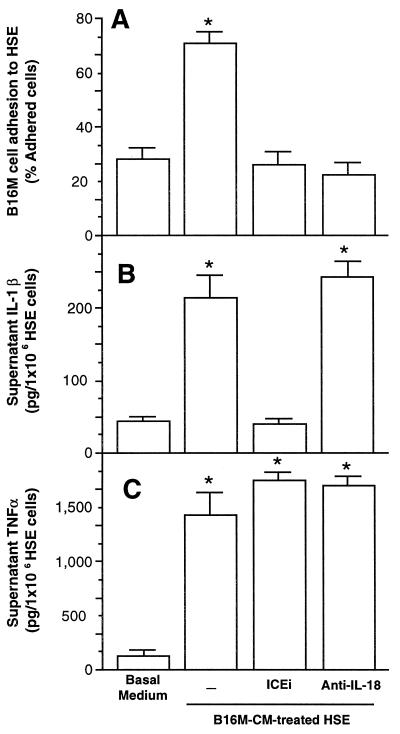
Figure 2.
Effect of B16M-CM on adhesion of B16M cells to HSE. Cultured HSE cells were incubated in the presence of B16M-CM for 10 h. (A) Percent of adhering B16M cells to HSE incubated with B16M-CM in the presence of ICEi (10 μM) or anti-IL-18 (10 μg/ml). (B) Level of IL-1β in HSE supernatants before adhesion assay. (C) Level of TNF-α in HSE supernatants before adhesion assay. After the removal of the HSE supernatants, the adhesion cell assay was performed as described in Materials and Methods. Data represent the means ± SD of four separate experiments performed by using four different preparations of HSE cells, each in six replicates (n = 24). ∗, P < 0.01 for the percentage of B16M cells adhering to B16M-CM-treated HSE and of IL-1β and TNF-α production with respect to the basal medium. The Student's two-tailed, unpaired t test was used.
In the next experiment shown in Fig. 3, exogenously added murine IL-1β did not reverse the effect of ICEi on HSE (Fig. 3A). This suggests that in addition to inhibiting the processing of the IL-1β precursor, the ICEi is also inhibiting the processing of the IL-18 precursor. Reverse transcription–PCR confirmed that HSE cells constitutively express IL-18 mRNA, as observed in murine spleen cells (20). The ability of murine IL-1β to directly increase the adherence of B16M cells is shown in Fig. 3B; however, in the presence of antimurine IL-18, this increase is reversed, again suggesting that IL-1β-induced IL-18 is increasing the expression of VCAM-1 on HSE. In a similar fashion, TNF-α added to HSE up-regulates melanoma cell adhesion (Fig. 3C), but this is reduced by the presence of anti-IL-18.
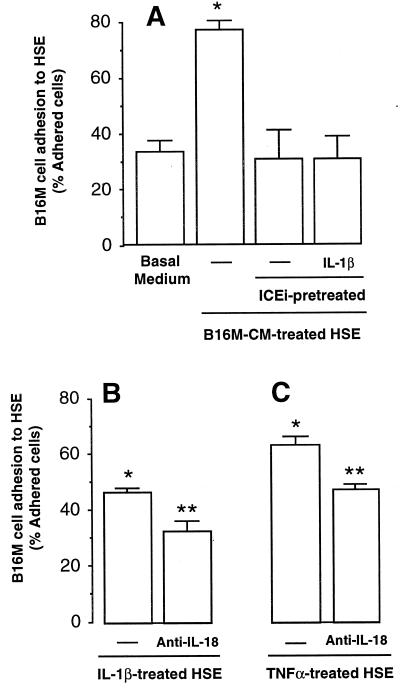
Figure 3.
B16M cell adhesion to cytokine-activated HSE. (A) HSE cells were incubated with basal medium or stimulated with B16M-CM for 8 h. ICEi (10 μM) was added to some wells 18 h before stimulation with B16M-CM. Recombinant murine IL-1β (1 ng/ml) was added together with B16M-CM. (B) The HSE was stimulated with 1 ng/ml murine IL-1β for 6 h with and without antimurine IL-18 IgG (10 μg/ml) added 1 h before the IL-1β. (C) The HSE was stimulated with 100 pg/ml murine TNF-α for 6 h with and without antimurine IL-18 IgG (10 μg/ml) added 1 h before the TNF-α. After each of the indicated incubation times, the adherence cell assay was performed as described in Materials and Methods. The results represent the means ± SD of three separate experiments, each in six replicates (n = 18). Differences in the percent of adhering cells with respect to untreated HSE (∗) and IL-1β- or TNF-α-treated HSE (∗∗) were statistically significant (P < 0.01) by the Student's two-tailed, unpaired t test.
The above data provide evidence that B16M-CM contains soluble factors that trigger a cascade in HSE in which the last steps leading to increased adhesiveness for melanoma cells involve release of biologically active IL-18. To demonstrate this, B16M-CM was incubated with HSE in the presence of recombinant human IL-18BP, which binds and neutralizes murine IL-18 (17). As shown in Table 2, IL-18BP prevents the adhesion of B16M melanoma cells induced by B16M-CM. The addition of IL-18BP to HSE reduced the percent of adhering cells from 35.1 to 8.7% (P < 0.01). This represents a 100% inhibition and is consistent with the high affinity binding of IL-18BP to IL-18 and complete neutralization of its biological activities at a molar excess of two (25). In fact, the number of adhering cells was below the number of adhering cells incubated with basal medium, suggesting IL-18BP suppression of constitutive as well as inducible IL-18 from HSE. These results demonstrate that IL-18-activated HSE is an essential component of adhesion of melanoma cells to HSE cells. In preliminary studies, in vivo administration of IL-18BP or anti-IL-18 antibodies before intrasplenic injection of B16M reduced the number of hepatic metastatic foci and metastatic density on day 10 by >50%.
Table 2.
Inhibitory effect of IL-18BP on B16M-CM-induced adhesion of B16M melanoma cells to hepatic sinusoidal endothelial cells
| % Melanoma cell adhesion* | |
|---|---|
| Basal medium | 10.15 ± 1.5 |
| B16M-CM | 35.10 ± 4.4 |
| B16M-CM + IL-18BP (1 ng/ml) | 15.00 ± 2.5† |
| B16M-CM + IL-18BP (10 ng/ml) | 8.70 ± 1.1† |
Means ± SD of two independent experiments performed in six replicates (n = 12 for each condition).
P < 0.01 with respect to B16-CM by ANOVA and the Scheffe F-test.
Direct evidence that IL-18 increases the expression of VCAM-1 on HSE is shown in Fig. 4. Murine IL-18 was incubated with HSE cells in the presence of TNFbp, IL-1Ra, or anti-VCAM-1. IL-18 increased the adhesiveness of B16M cells (P < 0.01) which was not affected by the presence of TNFbp or IL-1Ra, both present in high concentrations. However, anti-VCAM-1 completely reduced the IL-18-induced increase in the number of melanoma cells adhering to HSE cells. A nonspecific IgG did not affect the up-regulation of B16M cell adhesion to IL-18-treated HSE (data not shown).
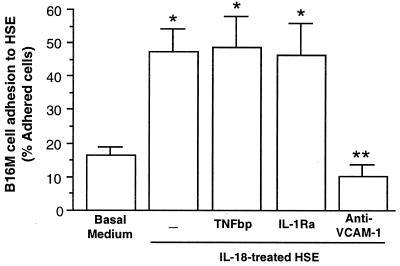
Figure 4.
Effect of anti-VCAM-1 on B16M cell adhesion to IL-18-treated HSE. HSE cells were incubated with 1 ng/ml recombinant murine IL-18 for 6 h. In some wells, 1 μg/ml TNFbp or 100 ng/ml IL-1Ra were added 10 min before IL-18. In other wells, 10 μg/ml anti-VCAM-1 antibody or 10 μg/ml anti-mouse IgG was added to HSE cells 30 min before the B16M cell adhesion assay. The percent of adhering B16M cells was determined as described in Materials and Methods. The results represent the means ± SD of three separate experiments, each in six replicates (n = 18). Differences in the percent of adhering cells with respect to basal medium-treated HSE (∗) or IL-18-treated HSE (∗∗) were statistically significant (P < 0.01 by the Student's two-tailed, unpaired t test).
Discussion
Inflammatory mechanisms facilitate cancer metastasis. Previous work has focused on IL-1 (4–6, 8, 26–30) and TNF (3, 31–33) as prometastatic cytokines. Although specific blockade of endogenous IL-1 and TNF reduce metastasis, other factors or cytokines, acting via the same or alternative pathways, are surely involved. In the present study, we have identified IL-18 as a proinflammatory cytokine with an unexpected pivotal position in the cytokine hierarchy that functions to increase metastatic spread in this model. We show that hepatic metastasis of intrasplenically injected B16M cells is dramatically reduced in IL-1β−/− and almost completely inhibited in ICE−/− mice. Splenic weights were not different, suggesting that the cytokine effects were at the level of hepatic implantation rather than on tumor growth at the injection site. Because ICE regulates the processing of both the IL-1β as well as the IL-18 precursors into biologically active molecules, the ICE−/− mice represent a quasi double knock-out mutant.
Although the present data showing reduced hepatic metastases in IL-1β-deficient mice confirm previous data demonstrating a role for IL-1 in hepatic spread of B16 (6, 34), those studies did not discriminate between a role for IL-1α or IL-1β. The present observation in IL-1β-deficient mice demonstrates that IL-1β and not IL-1α participates in the metastatic process. In fact, others have shown that IL-1α can protect against tumor growth (35). Similarly, IL-1β and not IL-1α is responsible for the development of the acute-phase response following turpentine-induced tissue damage and inflammation (36, 37).
From in vitro experiments, the data also indicate that the prometastatic effect of IL-18 depends on VCAM-1 expression, because VCAM-1 up-regulation accounts for all the adhesion-stimulating activity of B16M-CM-treated HSE. In this regard, preliminary studies by Western blot analysis confirm that recombinant murine IL-18 induces HSE expression of VCAM-1. In macrophagic cells, IL-18 increases expression of ICAM-1 (12). Thus, it is likely that IL-18-induced VCAM-1 expression itself can account for cancer cell adhesion to inflamed HSE and, hence, for inflammation-augmented hepatic metastases.
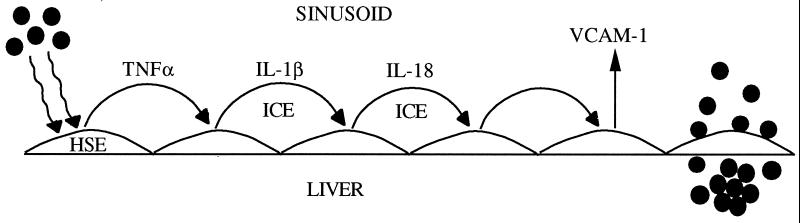
We also found that IL-18 neutralization did not reduce B16M-CM-induced TNF-α or IL-1β release from HSE, suggesting that their production was IL-18 independent. Conversely, neither TNFbp nor IL-1Ra was able to inhibit the increase in adhesiveness in IL-18-treated HSE, confirming that neither endogenous TNF nor IL-1 accounted for IL-18-induced HSE adhesiveness. This is in contrast to the effect of IL-18 in human peripheral blood mononuclear cells where the primary target cell of IL-18 is the non-CD14+ T and natural killer cell (38). These cells express receptors for IL-18, which, after stimulation by IL-18, release TNF. However, in the case of more typical reticuloendothelial cells such as the HSE cell, IL-18 is downstream from IL-1β and TNF. Thus, in HSE, a proinflammatory cytokine cascade exists in which TNF-α induces IL-1β; then, IL-1β, either alone or with TNF-α, induces IL-18 release. As such, TNF-α and IL-1β use the production of IL-18 to facilitate the increase in expression of VCAM-1 (Fig. 5).
Figure 5.
Proposed mechanism for B16M adhesion to HSE. From left, melanoma cells (●) release a prometastatic substance(s) that increases TNF-α production in HSE. TNF-α in turn stimulates IL-1β release via ICE and mature IL-1β stimulates IL-18 release, also via an ICE-dependent pathway. Mature IL-18 induces VCAM-1, which facilitates the adhesion of melanoma cells to HSE.
Unlike murine HSE, B16M cells did not constitutively express IL-18 mRNA as determined by reverse transcription–PCR, and incubation with ICEi for 18 h did not abrogate cytokine- and adhesion-stimulating activities of B16M-CM on HSE (data not shown). Therefore, there is no IL-18 in the B16M-CM to account for these findings. However, local production of IL-18 by HSE may arrest B16M cells during transit through the hepatic microvasculature. Additional findings were that B16M cells incubated with 1 ng/ml murine IL-18 for 6 h increased their adhesion to untreated HSE by twofold and increased their proliferation, suggesting that very late antigen-4 interaction was involved (data not shown). These findings are also in agreement with the reduction in metastasis volume observed in IL-1β−/− and ICE−/− mice (Table 1).
The role of IL-18 as an IFN-γ-inducing factor in the present study remains unknown. Following intrasplenic injection of 3 × 105 viable B16M cells, there was no increase in circulating IL-1β or IFN-γ in WT mice. However, IFN-γ plays both an agonist as well as an antagonist role in experimental models of various diseases (39). There is ample evidence that IFN-γ contributes to the immunostimulating, antitumor effects of several biological modifiers in murine cancer models. Indeed, the expression of mature IL-18 by genetically altered murine mammary carcinoma cells reduced the local growth of the tumor. This was caused by the production of IFN-γ (40). Because a reduction in IFN-γ production in ICE−/− mice is likely and since neutralization of IL-18 in vitro contributes to the findings of the present study, the role of IFN-γ in this model must be one of proinflammatory and not one of immunostimulation. IFN-γ is known to increase the expression of endothelial cell adhesion molecules.
IL-18BP is a unique, naturally occurring inhibitor of the biological activity of IL-18. IL-18BP resembles a soluble receptor for IL-18 but is unique in that it lacks a transmembrane and cytosolic domain (17). Recombinant IL-18BP completely reduced the adhesiveness of B16M-CM-stimulated HSE for melanoma cells, placing IL-18 in a strategic role for up-regulating the metastatic microenvironment of the liver sinusoids. IL-18BP is a constitutively expressed, circulating molecule but the levels of IL-18BP in patients with melanoma are presently unknown. Nevertheless, the balance between the naturally occurring agonist and antagonist may affect the outcome of melanoma implantation in the liver.
IL-18BP administration may also be useful in patients with melanoma or other cancer cells that employ VCAM-1-mediated adhesion to endothelia as a mechanism for invasion. However, IL-18 plays an essential role in regulating the production of the T-cell helper type 1 cytokine, IFN-γ. Several studies, in fact, show that administration of IFN-γ in various animal models of cancer growth serves to augment the immune response to the malignant cells. In fact, IL-18 has been used to increase the immune response against certain cancers in mice (41). Assuming the inhibition of IFN-γ production by IL-18BP does not impact on the immune response to the malignant cells, IL-18BP may be useful as adjuvant therapy in reducing adhesion of malignant cells to vascular endothelia, particularly in the liver.
Acknowledgments
We thank Interpharm Laboratories (Nes Ziona, Israel) for providing the IL-18BP. This work was supported in part by grants from the National Institutes of Health (AI-15614) and Colorado Cancer Center (CA-46934) to C.A.D, and by a grant from the National Institutes of Health (AI-2532359) to L.L.R. Grants from University of the Basque Country (EB214/95 and G17/98) and Comision Interministerial de Ciencia y Tecnologia (SAF99-0042) were to F.V-V. Fellowships from the Ministerio de Educación y Ciencia (Madrid) went to L.M., T.C., and M.A.A., and a fellowship from the University of the Basque Country was received by J.M.
Abbreviations
- IL-1β−/−
IL-1β deficient
- IL-1Ra
IL-1 receptor antagonist
- ICE
IL-1β-converting enzyme
- ICE−/−
ICE deficient
- ICEi
ICE inhibitor
- TNF
tumor necrosis factor
- TNFbp
TNF-binding protein
- IL-18BP
IL-18-binding protein
- HSE
hepatic sinusoidal endothelium
- B16M
B16 melanoma
- B16M-CM
B16M-conditioned medium
- VCAM-1
vascular cell adhesion molecule-1
- IFN-γ
interferon-γ
- WT
wild-type
References
- 1.Nicolson G L, Winkelhake J L. Nature (London) 1975;255:230–232. doi: 10.1038/255230a0. [DOI] [PubMed] [Google Scholar]
- 2.Rice G E, Bevilacqua M P. Science. 1989;246:1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- 3.Okahara H, Yagita H, Miyake K, Okumura K. Cancer Res. 1994;54:3233–3236. [PubMed] [Google Scholar]
- 4.Garofalo A, Chirivi R G S, Foglieni C, Pigot R, Mortarini R, Martin-Padura I, Anichini A, Gearing A J, Sanchez-Madrid F, Dejana E, et al. Cancer Res. 1995;55:414–419. [PubMed] [Google Scholar]
- 5.Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello C A. J Natl Cancer Inst. 1996;88:198–205. doi: 10.1093/jnci/88.3-4.198. [DOI] [PubMed] [Google Scholar]
- 6.Vidal-Vanaclocha F, Amézaga C, Asumendi A, Kaplanski G, Dinarello C A. Cancer Res. 1994;54:2667–2672. [PubMed] [Google Scholar]
- 7.Mendoza L, Olaso E, Anasagasti M J, Fuentes A, Vidal-Vanaclocha F. J Cell Physiol. 1998;174:322–330. doi: 10.1002/(SICI)1097-4652(199803)174:3<322::AID-JCP6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Anasagasti M J, Alvarez A, Martín J J, Mendoza L, Vidal-Vanaclocha F. Hepatology. 1997;25:840–846. doi: 10.1002/hep.510250410. [DOI] [PubMed] [Google Scholar]
- 9.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 10.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Nature (London) 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello C A. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 12.Kohka H, Yoshino T, Iwagaki H, Sakuma I, Tanimoto T, Matsuo Y, Kurimoto M, Orita K, Akagi T, Tanaka N. J Leukocyte Biol. 1998;64:519–527. doi: 10.1002/jlb.64.4.519. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto K, Okamura H, Nakanishi K, Akira S. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 16.Mendoza L, Carrascal T, Fuentes A, Anasagasti M J, Martin J J, DeLuca M, Dinarello C A, Vidal-Vanaclocha F. Proc Am Assoc Cancer Res. 1999;40:449. (abstr.). [Google Scholar]
- 17.Novick D, Kim S-H, Fantuzzi G, Reznikov L L, Dinarello C A, Rubinstein M. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 18.Engelmann H, Novick D, Wallach D. J Biol Chem. 1990;265:1531–1536. [PubMed] [Google Scholar]
- 19.Porat R, Paddock H N, Schwaitzberg S D, Connolly R J, Wilkens T, Dasch J R, Gascon M-P, Hutchison J S, Ythier A, Wallach D, et al. Crit Care Med. 1995;23:1080–1089. doi: 10.1097/00003246-199506000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Puren A J, Fantuzzi G, Dinarello C A. Proc Natl Acad Sci USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidal-Vanaclocha F, Rocha M, Asumendi A, Barbera-Guillem E. Hepatology. 1993;18:328–339. [PubMed] [Google Scholar]
- 22.Fantuzzi G, Puren A J, Harding M W, Livingston D J, Dinarello C A. Blood. 1998;91:2118–2125. [PubMed] [Google Scholar]
- 23.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann K, Conn C C, Siszynski D, Grabiec C, Trumbauer M A, Shaw A R, et al. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 24.Kuida K, Lippke J A, Ku G, Harding M W, Livingston D J, Su M S-S, Flavell R A. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 25.Kim, S.-H., Eisenstein, M., Reznikov, L. L., Fantuzzi, G., Novick, D., Rubinstein, M. & Dinarello, C. A. (2000) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 26.Lauri D, Bertomeu M-C, Orr F W. Clin Exp Metastasis. 1990;8:27–32. doi: 10.1007/BF00155590. [DOI] [PubMed] [Google Scholar]
- 27.Bani M R, Garofalo A, Scanziani E, Giavazzi R. J Natl Cancer Inst. 1991;83:119–123. doi: 10.1093/jnci/83.2.119. [DOI] [PubMed] [Google Scholar]
- 28.Bertomeu M C, Gallo S, Lauri D, Haas T A, Orr F W, Bastida E, Buchanan M R. Clin Exp Metastasis. 1993;11:243–250. doi: 10.1007/BF00121167. [DOI] [PubMed] [Google Scholar]
- 29.Burrows F J, Haskard D O, Hart I R, Marshall J F, Selkirk S, Poole S, Thorpe P E. Cancer Res. 1991;51:4768–4775. [PubMed] [Google Scholar]
- 30.Chirivi R G S, Garofalo A, Padura I M, Mantovani A, Giavazzi R. Cancer Res. 1993;53:5051–5054. [PubMed] [Google Scholar]
- 31.Malik S T, Naylor M S, East N, Oliff A, Balkwill F R. Eur J Cancer. 1990;26:1031–1034. doi: 10.1016/0277-5379(90)90044-t. [DOI] [PubMed] [Google Scholar]
- 32.Orosz P, Echtenacher B, Falk W, Ruschoff J, Weber D, Mannel D N. J Exp Med. 1993;177:1391–1398. doi: 10.1084/jem.177.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orosz P, Krüger A, Hubbe M, Rüschoff J, Von Hoegen P, MŠnnel D N. Int J Cancer. 1995;60:867–871. doi: 10.1002/ijc.2910600624. [DOI] [PubMed] [Google Scholar]
- 34.Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello C A. J Natl Cancer Inst. 1996;88:198–205. doi: 10.1093/jnci/88.3-4.198. [DOI] [PubMed] [Google Scholar]
- 35.Voronov E, Weinstein Y, Benharroch D, Cagnano E, Ofir R, Dobkin M, White R M, Zoller M, Barak V, Segal S, et al. Cancer Res. 1999;59:1029–1035. [PubMed] [Google Scholar]
- 36.Fantuzzi G, Ku G, Harding M W, Livingston D L, Sipe J D, Kuida K, Flavell R A, Dinarello C A. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 37.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puren A J, Fantuzzi G, Gu Y, Su M S-S, Dinarello C A. J Clin Invest. 1998;101:711–724. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Froyen G, Billiau F. Biotherapy. 1997;10:49–57. doi: 10.1007/BF02678217. [DOI] [PubMed] [Google Scholar]
- 40.Coughlin C M, Salhany K E, Wysocka M, Aruga E, Kurzawa H, Chang A E, Hunter C A, Fox J C, Trinchieri G, Lee W M F. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osaki T, Peron J M, Cai Q, Okamura H, Robbins P D, Kurimoto M, Lotze M T, Tahara H. J Immunol. 1998;160:1742–1749. [PubMed] [Google Scholar]