Abstract
The immediate-early protein IE1 is the principal transcriptional regulator of the baculovirus Autographa californica nucleopolyhedrovirus (AcMNPV). Transactivation by IE1 is dramatically stimulated by cis linkage of the affected promoter to AcMNPV homologous region (hr) elements that contain palindromic 28-bp repeats (28-mers) with enhancer activity. This hr-dependent transcriptional enhancement requires binding of the 28-mer by dimeric IE1. Here, we have defined IE1 domains required for this DNA binding in order to investigate the mechanism of IE1 function. Analysis of a panel of IE1 insertion mutations indicated that disruption of a highly conserved domain (residues 152 to 161) consisting of mostly positive-charged residues (basic domain I) abolished hr-dependent transactivation. Targeted mutagenesis of basic residues within basic domain I caused loss of hr-dependent transactivation but had no effect on IE1 oligomerization, nuclear localization, or hr-independent transactivation of viral promoters. Alanine substitutions of K152 and K154 or K160 and K161 impaired IE1 binding to 28-mer DNA as a homodimer, indicating that these basic residues are required for enhancer binding. Consistent with a DNA-binding defect, 28-mer interaction was improved by heterodimerization with wild-type IE1 or by increasing mutated IE1 concentrations. DNA binding mediated by basic domain I was also required for IE1 transactivation that occurred through physically separated, unlinked hr elements. We concluded that basic domain I is the enhancer-binding domain for IE1. Our data also suggest that DNA binding activates IE1 for transcriptional enhancement, possibly through a conformational change involving basic domain I.
The major transcriptional regulator of Autographa californica multiple nucleocapsid nucleopolyhedrovirus (AcMNPV) is the immediate-early protein IE1. Highly conserved among members of Baculoviridae, this 67-kDa DNA-binding protein is postulated to participate in critical processes during baculovirus infection, including the initiation of DNA replication and transregulation of transcription from early and late viral promoters (1, 13, 15, 17-20, 22, 23, 26, 28, 30, 34, 35, 39-41). Current evidence suggests that IE1's regulatory functions are mediated through its interaction with homologous region (hr) sequences that are repeated throughout the circular DNA genome of AcMNPV (reviewed in references 9 and 27). The baculovirus hrs are transcription enhancers and possible origins of DNA replication (11, 12, 14, 20, 22, 30, 37, 41, 42). In transfection assays, cis linkage of the hr element to baculovirus promoters can boost IE1-mediated transactivation as much as 200-fold (30, 39, 41). During infection, IE1 also colocalizes with viral DNA replication factories in the nucleus, where it may function as a DNA origin (hr)-binding protein (31, 35, 40). Despite these roles in transcription and DNA replication, the molecular mechanisms by which IE1 functions through DNA binding are unknown.
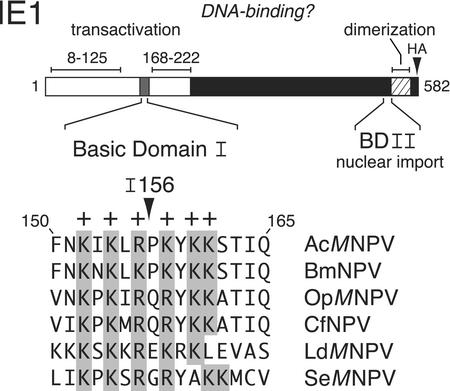
AcMNPV IE1 is a 582-residue nuclear phosphoprotein with separable domains that contribute to DNA binding and promoter transactivation (Fig. 1). The N-terminal half of IE1 contains two transcription stimulatory domains (residues 8 to 125 and 168 to 222), both composed of a high proportion of acidic residues (18, 43, 44). The transactivation domains are separated by a small but highly conserved domain (residues 152 to 161) which is enriched in basic residues and is thereby designated basic domain I (Fig. 1). At the C terminus, a smaller basic region designated basic domain II functions as the nuclear import element for IE1 (32). Immediately adjacent lies a helix-loop-helix-like domain (Fig. 1) that mediates IE1 dimerization, which is required for both nuclear import and DNA binding (32, 33, 43). Dimeric IE1 interacts directly with ∼28-bp imperfect palindromes (28-mers) that constitute the primary repetitive sequences within the AcMNPV hr enhancers (11, 20, 22, 42). The 28-mer is the minimal sequence motif required for IE1-mediated enhancer and origin-specific replication functions. Footprinting analyses and site-directed mutagenesis of the 28-mer suggest that the IE1 dimer contacts both half-sites of the 28-mer palindrome (20, 24, 33, 42). Although 28-mer binding by IE1 is required for hr-dependent transactivation (11, 12, 14, 18, 20, 22, 33, 42, 43), the IE1 residues responsible for 28-mer recognition are unknown.
FIG. 1.
Structure of IE1. (Top) IE1 functional domains. The 582-residue AcMNPV IE1 protein possesses two transactivation domains (open), from residues 8 to 125 and 168 to 222, which are separated by basic domain I. IE1 contains a nuclear import element (residues 543 to 568) within basic domain II (BDII) and a dimerization domain (hatched) from residues 543 to 568. The HA epitope (triangle) was inserted after IE1 residue 579. (Bottom) Basic domain I. AcMNPV IE1 residues 150 to 165 were aligned with the corresponding IE1 residues from the indicated group I and II nucleopolyhedroviruses. Conserved basic (+) residues are shaded. Insertion I156 contains four residues (GRSS) positioned after Arg156 (43).
Due to the importance of DNA binding for IE1 function, we have identified IE1 domains required for interaction with hr elements. To this end, we screened a panel of IE1 linker-scan insertion mutations (43) for their capacity to stimulate hr-dependent transactivation of viral promoters by using plasmid transfection assays. IE1I156, which carries a four-residue insert within basic domain I, was selectively impaired for hr-dependent transactivation. Subsequent site-directed mutagenesis indicated that basic domain I is required for hr-dependent transactivation and IE1 binding to the 28-mer element but is dispensable for hr-independent transactivation. The demonstrated participation of basic residues in the 28-mer interaction suggested for the first time that basic domain I is the hr DNA-binding domain (DBD) for IE1.
The mechanism by which DNA binding promotes IE1 transactivation is unknown. Binding to the 28-mer alone is insufficient for IE1-mediated enhancer activity (11, 24, 41). Thus, it has been postulated that a postbinding event is required for transcriptional stimulation by IE1. Such an event might include structural changes in the hr enhancer that are transmitted in cis to nearby promoters, a release of transcriptional repression, or a conformational change in IE1 that confers stimulatory activity. Consistent with the latter possibility, we show here that DNA binding is required for trans stimulation by IE1, which occurs through unlinked hr elements. Thus, transcription from promoters on a physically separate DNA molecule was stimulated only by hr binding competent IE1, not by basic domain I-mutated IE1. This finding suggests a new model wherein DNA binding activates IE1 for promoter stimulation, possibly through a conformational change involving basic domain I.
MATERIALS AND METHODS
Plasmids. (i) IE1 substitutions and deletions.
pIE1HA/BS used here for plasmid transfections encodes the wild-type, ie-1 gene under control of its own, full-length promoter with the hemagglutinin (HA) epitope inserted after IE1 residue 579 (33). The IE1 mutation pIE1I156HA/BS, which contains a four-residue insertion (GRSS) after residue 156, was generated by site-directed mutagenesis of pIE1BS (43) and subsequently cloned into pIE1HA/BS (33). The pairwise IE1 amino acid substitutions K152A/K154A (BI1), R156A/K158A (BI2), and K160A/K161A (BI3) were generated by site-directed mutagenesis of pIE1HA/BS using the complementary oligonucleotides 5′-GTG GTG GGC CAG TTT AAC GCA ATT GCA TTG AGG CC-3′ and 5′-GG CCT CAA TGC AAT TGC GTT AAA CTG GCC CAC CAC-3′, 5′-G GGC CAG TTT AAC AAA ATT AAG CTT GCG CCT GCA TAC AAG-3′ and 5′-CTT GTA TGC AGG CGC AAG CTT AAT TTT GTT AAA CTG GCC C-3′, and 5′-GG CCT AAA TAC GCG GCT AGC ACA ATT CAA AGC TGT GC-3′ and 5′-GC ACA GCT TTG AAT TGT GCT AGC CGC GTA TTT AGG CC-3′, respectively. The DraIII-NdeI fragment of each IE1 substitution K152A/K154A and K160A/K161A was subsequently inserted into pSP64/IE1 (43) for in vitro synthesis reactions. The substitutions K152A/K154A and K160A/K161A were combined to generate pIE1 (152/154/160/161)HA/BS (BI1:BI3) by inserting the StuI-NdeI fragment of pIE1 (160/161) HA/BS into the corresponding sites of pIE1 (152/154)HA/BS. The IE1 deletion mutation Δ150-162 (ΔβΙ) was created within pIE1HA/BS (33) by using the oligonucleotides 5′-GGC CAG TTT AAC AGC ACA ATT CAA AGC TGT GCA ACC-3′ and 5′-TTG AAT TGT GCT GTT AAA CTG GCC CAC CAC ACC TTG-3′. All mutations were verified by nucleotide sequence determination.
(ii) Luciferase and CAT reporters.
The hr-dependent reporter plasmid pBAS35K-Luc/28mer-up+/PA, containing the luciferase gene under control of the p35 basal promoter cis linked to a single copy of the 28-mer, and pFL35K-Luc/PA, containing the luciferase gene under control of the full-length p35 promoter, were described previously (33). The p39 upstream activating region (UAR)-dependent reporter plasmid p39K-Luc/PA was constructed by inserting the luciferase-encoding HindIII-MscI fragment from pGEM-luc (Promega) into the EcoRV and HindIII sites of p39Kprmtr/BS (38) and subsequently adding the polyadenylation signal from pIE1hr/PA (3). To generate pIE1-Luc/PA, the luciferase-encoding HindIII-SalI fragment of pGEM-luc (Promega) was inserted into the corresponding sites of the ie-1 promoter-containing plasmid pIE1hr/PA (3) after which the hr5 enhancer was excised. The reporter plasmids containing the chloramphenicol acetyltransferase (CAT) gene under control of the basal p35 promoter alone, p(Δ3′-162/Δ5′-30)35K-CAT (6), the basal p35 promoter with a single 28-mer, pBAS35K-CAT/28-mer-up+, and the complete hr5, pBAS35K-CAT/hr5-up+, were described previously (41).
Cells and plasmid transfection assays.
Spodoptera frugiperda IPLB-SF21 cells (45) (2 × 106/60-mm-diameter plate) propagated in TC100 growth medium (GIBCO Laboratories) supplemented with 2.6 mg of tryptose broth per ml and 10% heat-inactivated fetal bovine serum were transfected as described previously (33) by using N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl sulfate)-l-phosphatidylethanolamine, dioleoyl (C18:1, [cis]-9) and plasmid DNA in TC100. Cells were collected 48 h after transfection, washed with phosphate-buffered saline (21), and lysed by suspension in 250 μl of 1× cell culture lysis reagent (Promega). After clarification by centrifugation, luciferase activity was measured by using a luminometer (Monolight 3010). When purified luciferase (Promega) was used, the assay was linear from 104 to 108 relative light units (RLU). When CAT reporters were used, transfected cells were subjected to three freeze-thaw cycles, and clarified cell lysates (16,000 × g) were assayed for CAT activity by using [14C]chloramphenicol as described previously (30).
Cell fractionation.
SF21 cells (2 × 106/60-mm plate) were transfected with plasmid DNA (4 μg) encoding wild-type or mutated IE1HA. After 48 h, the cells were washed with phosphate-buffered saline (21) and a sample was taken for total protein (whole-cell fraction). Cells were lysed by suspension in TBN buffer (140 mM NaCl, 0.5% NP-40, 10 mM Tris [pH 6.5], 3 mM MgCl2, and protease inhibitors) and clarified by centrifugation (5,220 × g) for 5 min (4°C) as described previously (32). The supernatant was retained as the cytosolic fraction. The pellet was washed with TBN buffer, lysed by suspension in buffer A (40% glycerol, 4% sodium dodecyl sulfate [SDS], 3% dithiothreitol, 62.5 mM Tris; pH 6.8) for 15 min on ice, and forced through a 25-gauge needle to shear chromosomal DNA. After centrifugation (16,000 × g) for 10 min, the supernatant was retained as the nuclear fraction.
Electrophoretic mobility shift assays (EMSAs).
Wild-type and mutated IE1s were synthesized for 2 h at 30°C by using 30-μl coupled in vitro transcription-translation reaction mixtures (TNT system; Promega) programmed with plasmid DNA and SP6 RNA polymerase. 35S-labeled methionine-cysteine (NEN) was included only when levels of in vitro-synthesized protein were quantified by denaturing SDS-10% polyacrylamide gel electrophoresis. A 61-bp DNA probe containing the leftmost hr5 28-mer was generated by FokI and HinfI digestion of a PCR-derived fragment from plasmid phr5.1 (43). The agarose gel-purified DNA was dephosphorylated, end labeled with [γ-32P]ATP, and mixed with reticulocyte reactions containing in vitro-synthesized IE1. The resulting protein-DNA complexes were subjected to nondenaturing 5% polyacrylamide-Tris-glycine gel electrophoresis as previously described (42).
Immunoblot analysis.
Protein samples in 1% SDS-1% β-mercaptoethanol were electrophoresed on SDS-10% polyacrylamide gels. After protein transfer, nitrocellulose membranes were incubated with a 1:1,000 dilution of anti-HA (HA.11) (BAbCO) followed by alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Jackson ImmunoResearch Laboratories). Signal development was performed by using nitroblue tetrazolium chloride-5-bromo-4-chloro-3′-indolylphosphate p-toluidine salt colorimetric detection or the Western-Star chemiluminescent detection system (Tropix) as described previously (33).
Image processing.
Immunoblots were scanned at a resolution of 300 dots per inch by using a UMAX PowerLook III. The files were printed by using Adobe Photoshop.
RESULTS
Identification of an IE1 domain contributing to hr-dependent transactivation.
Previous studies using reporter plasmid transfection assays indicated that IE1 transactivates early baculoviral promoters when cis linked to hr enhancer elements or to the UAR of various baculoviral genes, which includes sequences 5′ to the RNA start site (reviewed in references 9 and 10). Whereas hr-mediated transactivation requires binding of IE1 to the hr 28-mer, no DNA-binding elements for IE1 have been identified for UAR-mediated transactivation. Due to this apparent difference in hr- and UAR-dependent transactivation, we postulated that the DBD of IE1 would be necessary for hr- but not UAR-dependent transactivation. To search for the hr-specific DBD, we therefore screened a panel of IE1 insertion mutations (43) for selective defects in hr-dependent transactivation.
By using the luciferase gene as a reporter in plasmid transfection assays, we identified insertion mutation IE1I156 (linker insertion after residue 156) as impaired for hr-dependent transactivation. IE1I156-mediated transactivation of an hr-dependent reporter containing a single copy of the 28-mer cis linked to the basal promoter of p35 (Fig. 2A) was reduced ≥55% compared to that by wild-type IE1 (Fig. 2C). In contrast, IE1I156 transactivation of UAR-dependent reporters containing either the full-length promoter of the early p39 or p35 genes (Fig. 2B) was comparable to that of wild-type IE1 (Fig. 2C). As expected, loss-of-function mutation IE1I553 (linker insertion after residue 553), which is defective for oligomerization and nuclear import (32, 33), failed to transactivate either hr- or UAR-dependent reporters (Fig. 2C).
FIG. 2.
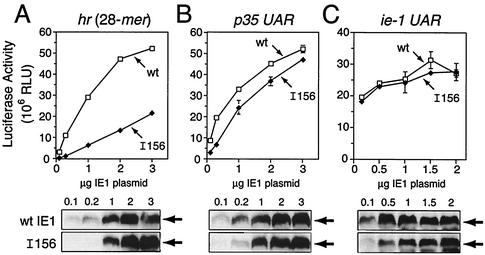
Differential hr- and UAR-dependent transactivation by IE1I156. (A) hr-dependent reporter plasmid. The luciferase gene was placed under control of the p35 basal promoter (TATA element and RNA start site, +1) which was cis linked to a single copy of the 28-mer from hr5. (B) UAR-dependent reporter plasmids. The luciferase gene was placed under control of either the p35 promoter with its UAR (nucleotides −110 to −30), the p39 promoter with its UAR (nucleotides −244 to −29), or the ie-1 promoter with its UAR (nucleotides −546 to −34). (C) Transactivation assays. SF21 cells (2 × 106/plate) were transfected with reporter plasmid (4 μg) alone or with reporter plasmid and plasmid (1 μg) encoding wild-type (wt) IE1, IE1I156, or IE1I553. Cell extracts were prepared 48 h later and assayed for luciferase activity. The values are averages ± standard deviations of triplicate transfections and are reported as RLU.
To confirm these findings and to assess the relative stability of IE1I156, we used dose-response transfection assays to compare IE1I156 with wild-type IE1. Transactivation of the hr (28-mer)-dependent reporter by wild-type IE1 was proportional to the level of IE1 produced (Fig. 3A). Although transactivation by IE1I156 also increased proportionally, the level of transactivation was consistently lower than that by wild-type IE1. At comparable levels of protein (using 1 μg of plasmid), IE1I156 transactivation was fivefold lower than that by wild-type IE1 (Fig. 3A). In contrast, transactivation of the p35 UAR-dependent reporter by IE1I156 was comparable to that by wild-type IE1 at all levels of protein (Fig. 3B). To determine if IE1I156 was impaired for activation of its own promoter, which might affect IE1 production, we assessed IE1I156 transactivation of a luciferase reporter driven by the full-length ie-1 promoter (Fig. 2B). At all levels of plasmid, transactivation of the ie-1 promoter by IE1I156 and wild-type IE1 were similar (Fig. 3C). Furthermore, steady-state levels of IE1I156 and wild-type IE1 were comparable at all but the lowest levels of plasmid. Collectively, these data indicated that insertion I156 selectively disrupted hr-dependent transactivation by IE1 without affecting protein stability or UAR-dependent transactivation.
FIG. 3.
Dose response of IE1I156 transactivation. SF21 cells (2 × 106/plate) were transfected with reporter plasmids containing the 28-mer (4 μg) (A), p35 UAR (4 μg) (B), or ie-1 UAR (2 μg) (C) alone or with increasing amounts (0.1 to 3 μg) of plasmid encoding C-terminal HA-tagged wild-type (wt) IE1 or IE1I156 (I156). Constant plasmid levels were maintained by supplementing with ie-1 promoter-containing plasmid pIE1-lacZ (2). Cell extracts were prepared 48 h later and assayed for luciferase activity. The values are averages ± standard deviations of triplicate transfections and are reported as RLU. (Bottom) Levels of wild-type (wt) IE1 or IE1I156 were determined 48 h after transfection by immunoblot analysis of total cell lysates (2.2 × 105 cell equivalent) using anti-HA.
Basic domain I residues are required for hr-dependent transactivation.
Arginine 156 is positioned in the middle of basic domain I, which contains the highest concentration of basic residues within baculovirus IE1s (Fig. 1). Since the DBD of transcriptional transactivators are often basic in nature (reviewed in references 29 and 36), we assessed the contribution of the basic residues comprising basic domain I towards IE1 function. To this end, we generated pairwise substitutions of K152/K154 (BI1), R156/K158 (BI2), K160/K161 (BI3), and K152/K154/K160/K161 (BI1:BI3) with alanine (Fig. 4A) and tested their effect on transactivation by using transfection assays (Fig. 4B). All substitutions, IE1BI1, IE1BI2, IE1BI3, and IE1BI1:BI3 transactivated the hr (28-mer)-dependent reporter at levels ≤30% of that by wild-type IE1. In contrast, transactivation of the UAR-dependent reporter by the IE1 mutations was ≥70% of wild-type IE1 (Fig. 4B). Confirming these results, an IE1 deletion lacking residues 150 to 161 (IE1ΔBI) was selectively impaired for hr-dependent transactivation (data not shown). For all basic domain I substitutions, the relative level of transactivation of the UAR-dependent promoter was 4.6- to 5.2-fold higher than that of the hr-dependent reporter (Fig. 4B). Since all IE1 substitutions were readily detected in transfected cells (Fig. 4C), the loss of hr-dependent transactivation was not due to IE1 instability. We concluded that the basic residues comprising basic domain I are required for hr-dependent but not UAR-dependent transactivation by IE1.
FIG. 4.
Comparison of hr- and UAR-dependent transactivation by basic domain I IE1 mutations. (A) Basic domain I. The indicated residues (arrows) within basic domain I (residues 152 to 161) were substituted with alanine and designated as indicated (underlined). Insertion I156 is shown. The residues deleted within IE1ΔBI are bracketed. (B) Luciferase reporter activities. SF21 cells were transfected with reporter plasmid (2 μg) (alone) or with reporter plasmid and plasmid (0.5 μg) encoding wild-type IE1HA or the indicated IE1HA mutation and assayed for luciferase activity as described in the legend to Fig. 2. The values for hr-dependent (solid bars) and p35 UAR-dependent (striped bars) transactivation are averages ± standard deviations of triplicate transfections. To calculate the fraction of activity, the level of UAR-dependent reporter activity was divided by the hr-dependent activity for each mutated IE1. (C) IE1HA levels. Total cell lysates (2 × 105 cell equivalent) were subjected to immunoblot analysis using anti-HA. Production of IE1HA was comparable in the presence of both reporter plasmids (data not shown).
Basic domain I is not required for IE1 nuclear localization.
Nuclear import elements for transcriptional activators are often comprised of basic residues (reviewed in reference 16). To test the potential role of basic domain I in nuclear entry, we compared subcellular localization of wild-type IE1 with that of basic domain I-mutated IE1s. As determined by detergent fractionation of transfected SF21 cells, wild-type IE1 and IE1I156 localized to the nucleus with comparable efficiencies (Fig. 5). Thus, IE1I156 exhibited normal nuclear localization, just like IE1BI1, IE1BI2, IE1BI3, and IE1ΔBI (32). Of these basic domain I mutations, only IE1BI1:BI3 exhibited a ∼15% reduction in nuclear import, which increased its cytosolic accumulation (Fig. 5). The integrity of our nuclear and cytoplasmic fractions was always verified by monitoring the distribution of nuclear immunophilin FKBP46 and cytosolic Op-IAPHA (data not shown), as previously reported (32). These data confirmed that basic domain I contributes little if any to the nuclear targeting of IE1.
FIG. 5.
Nuclear localization of IE1 mutations within basic domain I. SF21 cells were transfected with plasmids encoding wild-type (wt) IE1HA or the designated IE1HA mutation and fractionated 48 h later by using nonionic detergent and differential centrifugation. Samples (2 × 105 cell equivalent) of whole-cell lysate (W), cytosolic (C), and nuclear (N) fractions were subjected to immunoblot analysis using anti-HA.
IE1 basic domain I residues are required for hr binding.
Oligomerization is necessary but not sufficient for proper interaction of IE1 with the palindromic 28-mer enhancer (42). Our previous studies indicated that IE1I156 oligomerizes in vivo (33). Thus, to determine whether the defect in hr-dependent transactivation by IE1I156 is due to impaired DNA binding, we used EMSAs to evaluate IE1's interaction with the 28-mer present as a single copy in a DNA probe (Fig. 6A). Since Escherichia coli-generated IE1 is insoluble, we generated IE1 by using rabbit reticulocyte transcription-translation reactions. To assess homo- and heterodimerization, each mutated IE1 was cosynthesized in vitro with IE1Δ9-52, an oligomerization- and DNA-binding-competent form of IE1 with an electrophoretically distinct mobility (42, 43). As demonstrated by radiolabeling, the mutated IE1s were produced at levels comparable to that of IE1Δ9-52 (Fig. 6B). Wild-type IE1 and the basic domain I-mutated IE1s exhibited two mobilities, consistent with IE1 phosphorylation (4, 44), whereas IE1Δ9-52 and IE1I553 yielded single species (Fig. 6B).
FIG. 6.
DNA binding by IE1 mutations. (A) 28-mer-containing DNA probe. (Top) The 484-bp hr5 enhancer of AcMNPV possesses six 28-mers each bisected by an EcoRI (R) site. (Bottom) The 61-bp FokI-HinfI DNA probe (black highlight) was derived from the leftmost 28-mer (arrows). (B) In vitro IE1 synthesis. The indicated IE1 mutations were cosynthesized with IE1Δ9-52 in the presence of [35S]methionine-cysteine and subjected to SDS-polyacrylamide gel electrophoresis. Wild-type IE1 (wt), IE1Δ9-52 (Δ), and each mutation (mt) are indicated. (C) EMSAs. Wild-type IE1 or the indicated IE1 mutations were cosynthesized with IE1Δ9-52 in the absence of radiolabel, incubated with 32P-labeled 28-mer probe, and subjected to EMSA. Wild-type IE1 (wt IE1) and IE1Δ9-52 (IE1Δ) homodimeric complexes (wt:wt and Δ:Δ) are indicated (lanes 2 and 3). The expected mobility of mutant homodimers (mt:mt) and that of mutant-IE1Δ9-52 heterodimers (mt:Δ) are indicated at the right. DNA probe alone is shown in lane 1. (D) EMSAs with increasing IE1. IE1Δ9-52 (IE1Δ) was cosynthesized with increasing levels of IE1I156 (lanes 5 to 7), IE1BI1 (lanes 9 to 11), or IE1BI3 (lanes 13 to 15) and subjected to EMSA. The concentration of mutated IE1 increased fourfold over the range indicated. Homodimeric complexes (mt:mt) formed at the highest concentration of IE1I156, IE1BI1, and IE1BI3 in the absence of IE1Δ9-52 are shown (lanes 8, 12, and 16).
When wild-type IE1 and IE1Δ9-52 were synthesized independently and incubated with the 28-mer-containing probe, both proteins formed a single DNA complex in EMSAs (Fig. 6C, lanes 2 and 3). When cosynthesized, wild-type IE1 and IE1Δ9-52 formed three distinct complexes (Fig. 6C, lane 4) of decreasing size: a homodimer of wild-type IE1, a heterodimer of wild-type IE1 and IE1Δ9-52, and a homodimer of IE1Δ9-52. As a negative control, oligomerization-defective IE1I553 failed to bind the 28-mer or interact with IE1Δ9-52, as indicated by the absence of mutant homodimeric and mutant heterodimeric IE1Δ9-52 complexes (Fig. 6C, lane 8). Under these same conditions, IE1I156 and substitutions IE1BI1 and IE1BI3 failed to bind the 28-mer as a homodimer, as indicated by the absence of a mutant homodimer complex with a mobility comparable to that of the wild-type homodimer complex (lanes 5 to 7). Rather, IE1I156, IE1BI1, and IE1BI3 each formed a heterodimeric complex with IE1Δ9-52. These findings indicated that DNA binding but not oligomerization was impaired for these basic domain I-mutated IE1s.
To determine if disruption of basic domain I reduced the DNA-binding affinity of IE1, we determined the effect of increased IE1 concentrations on 28-mer binding efficiency (Fig. 6D). As the concentrations of IE1I156 (lanes 5 to 7), IE1BI1 (lanes 9 to 11), and IE1BI3 (lanes 13 to 15) were increased fourfold relative to IE1Δ9-52, discrete complexes corresponding to the homodimer of each mutated IE1 were detected in greater abundance. Furthermore, when the level of each mutated IE1 was increased fourfold in the absence of IE1Δ9-52, homodimers were readily detected (lanes 8, 12, and 16) but at levels dramatically lower than that for wild-type IE1 or IE1Δ9-52 (lanes 2 and 3). IE1I553 failed to bind DNA at all concentrations tested (data not shown). Collectively, these data indicated that disruption of basic domain I or substitution of these basic residues significantly reduced IE1's binding affinity for the 28-mer. Only upon heterodimerization with a DNA-binding-competent IE1 such as IE1Δ9-52 was indirect interaction with the 28-mer restored. We concluded that the basic residues of basic domain I are necessary for hr binding by IE1, as suggested by their requirement for hr-dependent transactivation.
Increased intracellular IE1 but not multiple 28-mers compensate for the hr-dependent deficiency in IE1I156.
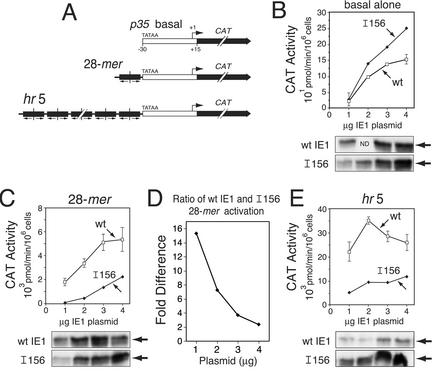
Since disruption of basic domain I impaired DNA binding by IE1, we predicted that increased IE1 concentrations would counteract the defect in 28-mer-dependent transactivation. We therefore compared the effect of increasing IE1I156 and wild-type IE1 levels on transactivation of a 28-mer-containing reporter gene. For these plasmid transfection assays, we used the p35 basal promoter linked to a CAT reporter (Fig. 7A). IE1I156 transactivation of the p35 basal promoter was greater than or equal to that by wild-type IE1 (Fig. 7B). Moreover, the steady-state levels of IE1I156 were comparable to that of wild-type IE1. Thus, IE1I156 exhibited higher than wild-type levels of transactivation of a basal promoter lacking cis-linked IE1-binding sites. However, upon cis linkage of a single copy of the 28-mer (Fig. 7A), transactivation by IE1I156 was significantly lower than that by wild-type IE1 (Fig. 7C), as expected. Despite this reduction, intracellular levels of wild-type and mutated IE1 were similar, except at the lowest plasmid concentration (Fig. 7C). Importantly, as indicated by plotting the difference in transactivation by IE1I156 and wild-type IE1 as a function of the amount of plasmid transfected (Fig. 7D), IE1I156's relative ability to transactivate increased from 2-fold to 16-fold as levels of IE1 protein increased. Thus, as the intracellular IE1 concentration increased relative to the number of available 28-mer binding sites, the defect in 28-mer-mediated transactivation by IE1I156 was mitigated.
FIG. 7.
Comparison of IE1I156 transactivation of 28-mer- and hr5-containing promoters. (A) Reporter plasmids. The CAT gene was placed under control of the basal p35 promoter (TATA element and RNA start site, +1) alone or cis linked to the 28-mer or the hr5 enhancer with its six 28-mers. (B, C, and E) CAT reporter activities. SF21 cells (2 × 106/plate) were transfected with reporter plasmid (4 μg) alone or with reporter plasmid and plasmid (1 to 4 μg) encoding wild-type (wt) IE1 or IE1I156. Plasmid levels were maintained by supplementing with pIE1-lacZ. Cell extracts were prepared 48 h later and assayed for CAT activity. The values for wild-type IE1 (wt [open boxes]) and IE1I156 (I156 [filled diamonds]) transactivation are averages ± standard deviations of triplicate transfections and are reported as the rate of [14C]chloramphenicol acetylation per 106 cells (in picomole per minute per 106 cells). IE1HA levels were assessed by immunoblot analysis of total cell lysates (2.2 × 105 cell equivalent) using anti-HA. ND, not determined. (D) Concentration effect on 28-mer transactivation by wild-type IE1 and IE1I156. The ratio of transactivation by wild-type IE1 and IE1I156 was calculated by dividing the reporter activity obtained for wild-type IE1 by that for IE1I156 and plotted as a function of the IE1 plasmid concentration used.
We also tested the effect of increasing the number of potential IE1 binding sites per molecule. As expected upon cis linkage of the hr5 enhancer with its six 28-mers, transactivation of the CAT reporter by wild-type IE1 (Fig. 7E) was ∼10-fold higher than that conferred by the 28-mer alone (Fig. 7C). Despite the increased number of 28-mers, transactivation of the hr5-containing reporter by IE1I156 was consistently lower than that by wild-type IE1 at all plasmid concentrations (Fig. 7E). Taking into account the higher steady-state levels of IE1I156 (Fig. 7E), the ability of IE1I156 to transactivate the hr5-linked reporter was significantly lower than that of wild-type IE1. Thus, the presence of multiple cis-linked 28-mers failed to compensate for the defect in DNA-dependent transactivation by IE1I156. Collectively, these data supported our conclusion that disruption of basic domain I selectively interferes with hr-dependent transactivation by IE1.
Basic domain I is required for hr-mediated enhancement in trans.
As shown previously (14), the AcMNPV hr elements can function in trans to stimulate IE1-mediated transactivation of viral promoters. The molecular mechanism of this lower-level trans effect is not fully understood but likely involves direct interaction of IE1 with hr-binding sites, including the 28-mer. Thus, to gain further insight into the role of basic domain I in IE1 function, we compared the effect of unlinked copies of the 28-mer on transactivation by IE1I156 and wild-type IE1. To this end, SF21 cells were cotransfected with (i) the 28-mer-linked p35 basal luc reporter (Fig. 2A), (ii) plasmid carrying only one copy of the 28-mer or the hr5 enhancer, and (iii) plasmid encoding either IE1I156 or wild-type IE1. By using this strategy, IE1 could interact with the unlinked 28-mers, the 28-mer-linked p35 basal reporter, or both (Fig. 8A).
FIG. 8.
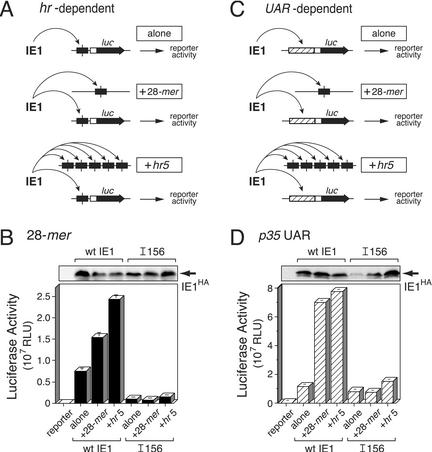
trans effects of hr elements on IE1 transactivation. (A) IE1 interactions during hr-dependent transactivation. SF21 cells were transfected with reporter plasmid (2 μg) containing the luciferase gene under control of the p35 basal promoter cis linked to the 28-mer (see Fig. 2A) and plasmid (0.5 μg) encoding wild-type (wt) IE1 or IE1I156. Cells were cotransfected with pBluescript alone, plasmid (2 μg) containing a single 28-mer, or plasmid (2 μg) containing hr5. As indicated (arrows), IE1 could interact with the 28-mer cis linked to the basal promoter (alone), the unlinked 28-mer (+28-mer), or the unlinked 28-mers of hr5 (+hr5). (B) 28-mer-dependent luciferase reporter activity. Extracts from cells (2 × 106/plate) were prepared 48 h after transfection and assayed for luciferase activity. The RLU values are averages ± standard deviations of triplicate transfections. IE1HA levels were assessed by immunoblot analysis of total cell lysates (2.2 × 105 cell equivalent) using anti-HA. (C) IE1 interactions during UAR-dependent transactivation. SF21 cells were transfected as described in the legend for panel A, except that the luciferase reporter was directed by the p35 UAR and basal promoter (see Fig. 2B). As indicated (arrows), IE1 could interact with UAR-specific factors (alone), the unlinked 28-mer (+28-mer), or the unlinked 28-mers of hr5 (+hr5). (D) UAR-dependent luciferase reporter activity. Luciferase activity and IE1HA levels were determined as described in the legend for panel B.
Transactivation of the 28-mer-linked basal reporter by wild-type IE1 increased by two- and threefold in the presence of unlinked 28-mer and hr5, respectively (Fig. 8B). Thus, the 28-mer itself is sufficient for trans stimulation of wild-type IE1. In contrast, neither the 28-mer nor the full-length hr5 enhancer stimulated transactivation by IE1I156 in trans. Rather, reporter activity with IE1I156 was similar in the presence or absence of unlinked 28-mer or hr5 and was eightfold lower than that mediated by wild-type IE1. Despite the lack of trans stimulation, intracellular IE1I156 levels were greater than or equal to that of wild-type IE1 (Fig. 8B). We concluded that basic domain I was required for trans stimulation by unlinked hr elements. Moreover, these findings supported our conclusion that disruption of basic domain I impairs hr-dependent transactivation by inhibiting the interaction of IE1 with the 28-mer.
To confirm these results and to gain further insight into the mechanism by which DNA binding by IE1 stimulates transcription, we tested the trans effect of unlinked 28-mers on UAR-dependent transactivation by IE1 (Fig. 8C). SF21 cells were transfected with (i) the p35 UAR-containing luc reporter, (ii) plasmid carrying only the 28-mer or hr5, and (iii) plasmid encoding IE1I156 or wild-type IE1. Unlinked 28-mer and hr5 stimulated wild-type IE1-mediated transactivation of the UAR-dependent promoter by six- and sevenfold, respectively (Fig. 8D). In the absence of 28-mer or hr5, transactivation by IE1I156 was comparable to that by wild-type IE1 (Fig. 8D). However, unlike wild-type IE1, transactivation by IE1I156 was unaffected by unlinked 28-mer or hr5. In the presence of hr5, IE1I156 transactivation increased less than twofold. However, this modest stimulation was attributed to the increased level of intracellular IE1I156 (Fig. 8D). We concluded that IE1's capacity to bind DNA is critical for the trans stimulatory activity of unlinked enhancer elements for both UAR- and hr-dependent promoters. Assuming that the hr-containing molecules remained physically separated from the affected promoters during the course of transfection, these findings suggested for the first time that DNA binding enhances the transactivation potency of IE1 (see below).
DISCUSSION
By screening a panel of random insertion mutations and subsequently using site-specific mutagenesis, we have determined that IE1's lysine-rich basic domain I is required for hr enhancer binding and hr-dependent transactivation (Fig. 9A). Furthermore, the demonstrated role of individual basic residues comprising basic domain I (residues 152 to 161) in mediating interaction with hr nucleotide sequences suggests that this highly conserved domain is the principal DBD of IE1. Our data support a model in which basic domain I functions to concentrate IE1 at the hr enhancers to stimulate transcription by a DNA-binding-dependent mechanism. By virtue of its hr-binding function, we predict that basic domain I is also required for IE1's role in DNA replication (17, 35, 40).
FIG. 9.
Model for DNA-binding-induced IE1 activation. (A) Charged domains within IE1. Basic domain I (BDI) (shaded) with its pI of 11.5 is embedded between the two acidic transactivation domains, designated Trans I (pI 3.7) and Trans II (pI 4.3). At the C terminus, basic domain II (BDII) mediates nuclear import. (B) Model for DNA-induced IE1 conformational changes. In the absence of DNA, basic domain I (positive charges) interacts with the acidic transactivation domains (negative charges), repressing their activities. Upon DNA binding, basic domain I preferentially interacts with its 28-mer recognition sequence, pulling away from the transactivation domains and relieving transcriptional repression. (C) Model for 28-mer interaction by dimeric IE1. Each 28-mer half-site (hatched arrows) is contacted by the exposed basic domain I of an IE1 monomer, which also interacts with its symmetric partner by dimerization involving the C terminus.
Participation of basic domain I in hr enhancer binding.
DNA binding is required for hr-mediated enhancement of transactivation by IE1 (18, 22, 33, 42, 43). Thus, our finding that IE1I156 was selectively impaired for hr (28-mer)-mediated transactivation indicated that basic domain I participates directly or indirectly in DNA binding by IE1 (Fig. 2). This conclusion was supported by the normal transactivation of UAR-dependent promoters (p35, p39, and ie-1) by IE1I156 (Fig. 2 and 3). Moreover, disruption of basic domain I had no effect on nuclear import or oligomerization of IE1 (Fig. 5 and 6). Thus, it is unlikely that basic domain I mutations caused significant misfolding of IE1.
Our data support a direct role for basic domain I in hr enhancer-specific DNA binding. Most important, alanine substitutions of the basic residues comprising basic domain I not only inhibited hr-mediated transactivation but also impaired hr binding. As determined by EMSAs (Fig. 6), pairwise substitution of each of the basic residues severely reduced binding of homodimeric IE1 to 28-mer-containing DNA probes. Thus, all of the basic residues of basic domain I are responsible for stable interaction with the hr 28-mer. It is noteworthy that basic domain I contains the highest concentration of basic residues within IE1 and that each residue (five lysines and an arginine) is highly conserved among baculovirus IE1s (Fig. 1). Indeed, the DBDs of many oligomeric transactivators, including basic helix-loop-helix and basic leucine zipper proteins, are comprised of lysine and arginine residues that make direct contact with the cognate DNA-binding site (reviewed in references 7, 25, 29, and 36).
In addition, 28-mer binding by basic domain I-disrupted IE1 was concentration dependent. Consistent with a decreased binding constant for DNA, the low level of DNA binding by IE1I156 and basic residue substitutions IE1BI1 and IE1BI3 was increased at higher concentrations of IE1 (Fig. 6). Furthermore, hr-mediated transactivation by IE1I156 was more concentration dependent than that by wild-type IE1. In particular, increasing the concentration of IE1I156 boosted the relative level of 28-mer-linked transactivation by eightfold above that for wild-type IE1 over the same range (Fig. 7D). Thus, our data are most consistent with the conclusion that basic domain I constitutes the hr-specific DBD for IE1. Direct proof awaits structural determination of the IE1-DNA (28-mer) complex.
Are there other IE1 domains that participate in 28-mer binding? Basic domain II (Fig. 9A), which is the nuclear import element for IE1 (32), has a position analogous to the DBD of basic helix-loop-helix transactivators. Mutagenesis of basic domain II eliminated 28-mer binding by IE1 (43), but it also impaired oligomerization which is required for DNA binding (33). Although a direct role of basic domain II in DNA binding remains to be ruled out, our findings suggest that IE1 has a structural organization that is very distinct from that of the basic helix-loop-helix activators (29, 36). Our recent studies have also indicated that other IE1 domains participate indirectly in DNA binding by contributing to IE1's folding (B. Y. Liu and P. D. Friesen, unpublished data).
Role of DNA binding: insight through hr-mediated trans stimulation.
Confirming earlier studies (14), IE1 transactivation was stimulated by unlinked copies of hr enhancer elements (Fig. 8). Here, we showed that unlinked plasmids carrying either the 28-mer or the full-length hr5 enhancer boosted transactivation of a 28-mer-dependent reporter by wild-type IE1, but not by DNA-binding-defective IE1I156 (Fig. 8B). Thus, DNA binding was required for trans stimulation by unlinked hr enhancers. Unexpectedly, trans stimulation by the 28-mer and hr5 was even higher for p35 UAR-dependent transcription, ranging from six- to sevenfold, respectively (Fig. 8D). Since this trans stimulation did not occur in the presence of IE1I156, DNA binding was also required for UAR-dependent promoters. Thus, hr-specific interaction by IE1 is a critical step in the mechanism of trans stimulation and provides new insight into IE1 mechanisms (see below). IE1 can promote recombination between transfected plasmid DNAs (5). Although the physical status of transfected plasmids in SF21 cells is unknown, it is unlikely that recombinational acquisition of enhancer DNA by our plasmid reporters was responsible for the stimulatory effect. In particular, we have shown previously that cis linkage of hr5 boosts expression from the p35 UAR by 18-fold in the presence of IE1 (30). Thus, if insertion of hr5 into the p35 UAR reporter plasmid were to fully account for the 7-fold stimulation, then ∼40% of the reporter plasmids (7-fold trans effect divided by the 18-fold cis effect) must have acquired hr5 by recombination. This high rate of recombination is unlikely (5). Moreover, increasing the hr- or 28-mer-containing plasmid levels in the presence of limiting IE1 did not increase the trans stimulatory effect, which is inconsistent with stimulation by recombination (data not shown).
Model for IE1 activation upon DNA binding.
The most straightforward explanation for the DNA-binding-dependent trans stimulation by hr elements is that IE1 is activated upon 28-mer binding and that this “energized” IE1 stimulates transcription from both linked and unlinked promoters. Thus, our data suggest a new model in which DNA binding induces a change in IE1, probably in conformation, that increases its capacity to stimulate transcription from viral promoters (Fig. 9). The unusual location of the DBD of basic domain I, which as shown here is embedded between IE1's two transactivation domains, provides provocative clues as to the molecular mechanism of this DNA-induced activation. Basic domain I (pI of 11.5) is sandwiched between the left (Trans I) and right (Trans II) transactivation domains with acidic pIs of 3.7 (∼100 residues) and 4.3 (∼50 residues), respectively (Fig. 9A). Basic domain I's strikingly opposite charge suggests that it interacts with the adjacent transactivation domains to mask or neutralize them (Fig. 9B). Indeed, the acidic nature of the transactivation domains is conserved among baculovirus IE1s and is required for transcriptional stimulation (8). However, upon direct interaction with negatively charged 28-mer DNA, basic domain I may pull away from the transactivation domains (Fig. 9B), thereby unmasking them for direct interaction with the cellular transcription machinery required for transcriptional stimulation. Since this conformational change must occur in the context of an IE1 dimer, each basic domain I would make contact with a 28-mer half-site such that the IE1 dimer exposes its acidic domains for maximal transactivation while maintaining its C-terminal oligomeric interactions (Fig. 9C). This DNA-specific mechanism for induced activation is attractive, since it would promote specificity of IE1 transactivation (i.e., IE1 is activated only upon interaction with virus-specific hr sequences) and thereby reduce nonspecific, promiscuous activation of nonviral promoters.
Our data combined with those of others provide strong support for this model of DNA-induced IE1 activation. First, upon binding to hr elements, IE1 stimulated transcription from unlinked promoters at a level significantly higher than that when unbound (Fig. 8). Second, DNA binding was required for this activation, since 28-mer-binding-deficient IE1I156 was not activated by unlinked hr elements (Fig. 8). Third, in independent assays where IE1 was fused to the E. coli lac repressor (44), basic domain I had no transcription stimulatory activity on its own but exerted a pronounced negative effect on transcriptional activation of the Trans I domain and a smaller negative effect on the Trans II domain. Although the stimulatory activity of Trans II remains to be demonstrated within native IE1, our independent studies using Gal4-IE1 fusions confirmed that basic domain I suppressed Trans II (43). Thus, in the absence of 28-mer binding, basic domain I represses the transactivation potential of both acidic domains, a finding consistent with domain masking (see above). According to this model, disruption of basic domain I should increase the transactivation potential of IE1 for promoters lacking hr sequences due to loss of the inhibitory activity of basic domain I. Consistent with this prediction, basic domain I-disrupted IE1I156 exhibited higher transactivation of the p35 basal promoter compared to that by wild-type IE1 (Fig. 7B). Lastly, it is relevant that the N-terminal domain of IE1 from baculovirus OpMNPV has a structural organization analogous to that of AcMNPV IE1. Basic domain I of OpMNPV IE1 is nearly identical to that of AcMNPV IE1 and splits two acidic domains (∼100 and 50 residues) with pIs of 4.0 and 4.3, respectively. The leftmost acidic domain has transactivation potential (8), even though its amino acid sequence varies considerably from that of AcMNPV IE1. This striking similarity in structural organization argues that the transactivation domains of both IE1s are regulated by a common mechanism.
Conformational changes of basic helix-loop-helix transcriptional activators upon binding to their DNA recognition sites are well documented (36). Indeed, on the basis that IE1 binding to a single 28-mer half-site is insufficient for transcriptional stimulation, it has been postulated that a molecular event subsequent to DNA binding is required for IE1 transactivation (11, 22, 41). A conformational change in IE1 induced by simultaneous interaction of basic domain I with both 28-mer half-sites (Fig. 9C) is consistent with such a molecular event. Further studies are required to test this model and define the molecular nature of DNA-binding-induced conformational changes of IE1.
Acknowledgments
This work was supported in part by Public Health Service grant AI25557 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Carson, D. D., M. D. Summers, and L. A. Guarino. 1991. Transient expression of the Autographa californica nuclear polyhedrosis virus immediate-early gene, IE-N, is regulated by three viral elements. J. Virol. 65:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartier, J. L. 1994. Master's thesis. University of Wisconsin—Madison, Madison.
- 3.Cartier, J. L., P. A. Hershberger, and P. D. Friesen. 1994. Suppression of apoptosis in insect cells stably transfected with baculovirus p35: dominant interference by N-terminal sequences p351-76. J. Virol. 68:7728-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, J., and L. A. Guarino. 1995. Expression of the IE1 transactivator of Autographa californica nuclear polyhedrosis virus during viral infection. Virology 209:99-107. [DOI] [PubMed] [Google Scholar]
- 5.Crouch, E. A., and A. L. Passarelli. 2002. Genetic requirements for homologous recombination in Autographa californica nucleopolyhedrovirus. J. Virol. 76:9323-9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickson, J. A., and P. D. Friesen. 1991. Identification of upstream promoter elements mediating early transcription from the 35,000-molecular-weight protein gene of Autographa californica nuclear polyhedrosis virus. J. Virol. 65:4006-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flint, J., and T. Shenk. 1997. Viral transactivating proteins. Annu. Rev. Genet. 31:177-212. [DOI] [PubMed] [Google Scholar]
- 8.Forsythe, I. J., C. E. Shippam, L. G. Willis, S. Stewart, T. Grigliatti, and D. A. Theilmann. 1998. Characterization of the acidic domain of the IE1 regulatory protein from Orgyia pseudotsugata multicapsid nucleopolyhedrovirus. Virology 252:65-81. [DOI] [PubMed] [Google Scholar]
- 9.Friesen, P. D. 1997. Regulation of baculovirus early gene expression, p. 141-166. In L. K. Miller (ed.), The baculoviruses. Plenum Publishing Corporation, New York, N.Y.
- 10.Friesen, P. D., and L. K. Miller. 2001. Insect viruses, p. 599-628. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 11.Guarino, L. A., and W. Dong. 1994. Functional dissection of the Autographa californica nuclear polyhedrosis virus enhancer element hr5. Virology 200:328-335. [DOI] [PubMed] [Google Scholar]
- 12.Guarino, L. A., M. A. Gonzalez, and M. D. Summers. 1986. Complete sequence and enhancer function of the homologous DNA regions of Autographa californica nuclear polyhedrosis virus. J. Virol. 60:224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarino, L. A., and M. D. Summers. 1986. Functional mapping of a trans-activating gene required for expression of a baculovirus delayed-early gene. J. Virol. 57:563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarino, L. A., and M. D. Summers. 1986. Interspersed homologous DNA of Autographa californica nuclear polyhedrosis virus enhances delayed-early gene expression. J. Virol. 60:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarino, L. A., and M. D. Summers. 1987. Nucleotide sequence and temporal expression of a baculovirus regulatory gene. J. Virol. 61:2091-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaffman, A., and E. K. O'Shea. 1999. Regulation of nuclear localization: a key to a door. Annu. Rev. Cell Dev. Biol. 15:291-339. [DOI] [PubMed] [Google Scholar]
- 17.Kool, M., C. H. Ahrens, R. W. Goldbach, G. F. Rohrmann, and J. M. Vlak. 1994. Identification of genes involved in DNA replication of the Autographa californica baculovirus. Proc. Natl. Acad. Sci. USA 91:11212-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs, G. R., J. Choi, L. A. Guarino, and M. D. Summers. 1992. Functional dissection of the Autographa californica nuclear polyhedrosis virus immediate-early 1 transcriptional regulatory protein. J. Virol. 66:7429-7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs, G. R., L. A. Guarino, and M. D. Summers. 1991. Novel regulatory properties of the IE1 and IE0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J. Virol. 65:5281-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremer, A., and D. Knebel-Mörsdorf. 1998. The early baculovirus he65 promoter: on the mechanism of transcriptional activation by IE1. Virology 249:336-351. [DOI] [PubMed] [Google Scholar]
- 21.Lee, H. H., and L. K. Miller. 1978. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J. Virol. 27:754-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leisy, D. J., C. Rasmussen, H. T. Kim, and G. F. Rohrmann. 1995. The Autographa californica nuclear polyhedrosis virus homologous region 1a: identical sequences are essential for DNA replication activity and transcriptional enhancer function. Virology 208:742-752. [DOI] [PubMed] [Google Scholar]
- 23.Leisy, D. J., C. Rasmussen, E. O. Owusu, and G. F. Rohrmann. 1997. A mechanism for negative gene regulation in Autographa californica multinucleocapsid nuclear polyhedrosis virus. J. Virol. 71:5088-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leisy, D. J., and G. F. Rohrmann. 2000. The Autographa californica nucleopolyhedrovirus IE-1 protein complex has two modes of specific DNA binding. Virology 274:196-202. [DOI] [PubMed] [Google Scholar]
- 25.Littlewood, T. D., and G. I. Evan. 1995. Transcription factors 2: helix-loop-helix. Protein Profile 2:621-702. [PubMed] [Google Scholar]
- 26.Lu, A., and E. B. Carstens. 1993. Immediate-early baculovirus genes transactivate the p143 gene promoter of Autographa californica nuclear polyhedrosis virus. Virology 195:710-718. [DOI] [PubMed] [Google Scholar]
- 27.Lu, A., P. J. Krell, J. M. Vlak, and G. F. Rohrmann. 1997. Baculovirus DNA replication, p. 171-191. In L. K. Miller (ed.), The baculoviruses. Plenum Publishing Corporation, New York, N.Y.
- 28.Lu, A., and L. K. Miller. 1995. The roles of eighteen baculovirus late expression factor genes in transcription and DNA replication. J. Virol. 69:975-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massari, M. E., and C. Murre. 2000. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20:429-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissen, M. S., and P. D. Friesen. 1989. Molecular analysis of the transcriptional regulatory region of an early baculovirus gene. J. Virol. 63:493-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okano, K., V. S. Mikhailov, and S. Maeda. 1999. Colocalization of baculovirus IE-1 and two DNA-binding proteins, DBP and LEF-3, to viral replication factories. J. Virol. 73:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson, V. A., J. A. Wetter, and P. D. Friesen. 2002. Baculovirus transregulator IE1 requires a dimeric nuclear localization element for nuclear import and promoter activation. J. Virol. 76:9505-9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson, V. A., J. A. Wetter, and P. D. Friesen. 2001. Oligomerization mediated by a helix-loop-helix-like domain of baculovirus IE1 is required for early promoter transactivation. J. Virol. 75:6042-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passarelli, A. L., and L. K. Miller. 1993. Three baculovirus genes involved in late and very late gene expression: ie-1, ie-n, and lef-2. J. Virol. 67:2149-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathakamuri, J. A., and D. A. Theilmann. 2002. The acidic activation domain of the baculovirus transactivator IE1 contains a virus-specific domain essential for DNA replication. J. Virol. 76:5598-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patikoglou, G., and S. K. Burley. 1997. Eukaryotic transcription factor-DNA complexes. Annu. Rev. Biophys. Biomol. Struct. 26:289-325. [DOI] [PubMed] [Google Scholar]
- 37.Pearson, M. N., R. M. Bjornson, G. D. Pearson, and G. F. Rohrmann. 1992. The Autographa californica baculovirus genome: evidence for multiple replication origins. Science 257:1382-1384. [DOI] [PubMed] [Google Scholar]
- 38.Pullen, S. S. 1996. Ph.D. thesis. University of Wisconsin—Madison, Madison.
- 39.Pullen, S. S., and P. D. Friesen. 1995. Early transcription of the ie-1 transregulator gene of Autographa californica nuclear polyhedrosis virus is regulated by DNA sequences within its 5′ noncoding leader region. J. Virol. 69:156-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapp, J. C., J. A. Wilson, and L. K. Miller. 1998. Nineteen baculovirus open reading frames, including LEF-12, support late gene expression. J. Virol. 72:10197-10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodems, S. M., and P. D. Friesen. 1993. The hr5 transcriptional enhancer stimulates early expression from the Autographa californica nuclear polyhedrosis virus genome but is not required for virus replication. J. Virol. 67:5776-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodems, S. M., and P. D. Friesen. 1995. Transcriptional enhancer activity of hr5 requires dual-palindrome half sites that mediate binding of a dimeric form of the baculovirus transregulator IE1. J. Virol. 69:5368-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodems, S. M., S. S. Pullen, and P. D. Friesen. 1997. DNA-dependent transregulation by IE1 of Autographa californica nuclear polyhedrosis virus: IE1 domains required for transactivation and DNA binding. J. Virol. 71:9270-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slack, J. M., and G. W. Blissard. 1997. Identification of two independent transcriptional activation domains in the Autographa californica multicapsid nuclear polyhedrosis virus IE1 protein. J. Virol. 71:9579-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaughn, J. L., R. H. Goodwin, G. L. Thompkins, and P. McCawley. 1977. Establishment of two insect cell lines from the insect Spodoptera frugiperda (Lepidoptera:Noctuidae). In Vitro 13:213-217. [DOI] [PubMed] [Google Scholar]