Abstract
We present the microbiological and molecular characterization of bacteria isolated from four chimpanzees and one gorilla thought to have died of an anthrax-like disease in Côte d'Ivoire and Cameroon. These isolates differed significantly from classic Bacillus anthracis by the following criteria: motility, resistance to the gamma phage, and, for isolates from Cameroon, resistance to penicillin G. A capsule was expressed not only after induction by CO2 and bicarbonate but also under normal growth conditions. Subcultivation resulted in beta-hemolytic activity and gamma phage susceptibility in some subclones, suggesting differences in gene regulation compared to classic B. anthracis. The isolates from Côte d'Ivoire and Cameroon showed slight differences in their biochemical characteristics and MICs of different antibiotics but were identical in all molecular features and sequences analyzed. PCR and Southern blot analyses confirmed the presence of both the toxin and the capsule plasmid, with sizes corresponding to the B. anthracis virulence plasmids pXO1 and pXO2. Protective antigen was expressed and secreted into the culture supernatant. The isolates possessed variants of the Ba813 marker and the SG-749 fragment differing from that of classic B. anthracis strains. Multilocus sequence typing revealed a close relationship of our atypical isolates with both classic B. anthracis strains and two uncommonly virulent Bacillus cereus and Bacillus thuringiensis isolates. We propose that the newly discovered atypical B. anthracis strains share a common ancestor with classic B. anthracis or that they emerged recently by transfer of the B. anthracis plasmids to a strain of the B. cereus group.
Bacillus anthracis, the etiological agent of anthrax, is found worldwide and is able to infect virtually all mammals, including humans. The danger of its intentional release is prevalent since the anthrax attacks in the United States in 2001.
Together with Bacillus cereus and Bacillus thuringiensis, B. anthracis is a member of the Bacillus cereus group. B. thuringiensis is an insect pathogen, and B. cereus is known mainly as a food poisoning pathogen characterized by toxin-induced emetic and diarrheagenic syndromes. More severe infections develop mainly in immunocompromised patients or patients with other risk factors (for a review, see reference 12). However, life-threatening and fatal cases of pneumonia and bacteremia resembling infection by B. anthracis were also observed in otherwise healthy people (20, 39).
Multilocus sequence typing (MLST) and fluorescent amplified fragment length polymorphism (AFLP) have proved their efficiency in typing members of the B. cereus group. Both methods show that genetic diversity is high within the B. cereus and B. thuringiensis groups, whereas B. anthracis is highly homogenous and can therefore be considered a particularly monomorphic species (17, 19, 45). Typing of B. anthracis strains and isolates is usually achieved by multiple-locus variable-number tandem repeat analysis (MLVA) (25). However, based on the chromosome genomic comparison reviewed previously by Rasko et al. (48), it is not possible to distinguish members of the B. cereus group from one another, and therefore B. anthracis, B. cereus, and B. thuringiensis can be considered one species (16). The differences in pathogenicity among the three species are mainly encoded on plasmids. B. anthracis possesses two plasmids, pXO1 and pXO2, both essential for virulence, that carry genes for toxin synthesis (pag, cya, and lef) and capsule synthesis (capB, capC, capA, and capD), respectively (37, 41). Regulation of virulence gene expression is different in B. anthracis and in other B. cereus group members (1, 28, 38, 42, 56).
Fast and reliable diagnosis of B. anthracis is of high importance for timely and adequate treatment of a patient. So far, B. anthracis strains were easily distinguished from nonanthrax members of the B. cereus group by a few microbiological tests that are recommended by the World Health Organization (WHO) and by the Centers for Disease Control and Prevention (CDC); in contrast to B. cereus and B. thuringiensis, B. anthracis is nonmotile, lacks beta-hemolytic activity, and is sensitive to penicillin G and to lysis by the gamma phage. It is able to produce a capsule in vivo and in vitro under appropriate conditions (53). However, not a single diagnostic trait appears to be consistent for all B. anthracis isolates (6, 30, 36), and atypical isolates of B. anthracis and other members of the B. cereus group have been described previously (26). Therefore, the application of molecularly based methods like PCR has become increasingly important for the diagnosis of B. anthracis (9, 14, 43, 46).
Anthrax is globally distributed, but the most diverse isolates are found in southern Africa, resulting in speculations that Africa is the origin of B. anthracis (24). Herbivorous animals are the most susceptible animals, and ungulates of the savannahs are most frequently affected. Cases in primates, except humans, were rarely observed (11). Therefore, it was exceptional to find wild great apes in rainforest regions that had apparently died from anthrax. It was first described in the Taï National Park, Côte d'Ivoire, where at least six wild chimpanzees died of an acute bacterial infection between October 2001 and June 2002. Sequencing of the 16S rRNA gene and real-time PCR using appropriate genome regions indicated the presence of a member of the B. cereus group that possessed the plasmid-encoded virulence genes of B. anthracis (31). At the end of 2004, more cases of anthrax among great apes were diagnosed: three chimpanzees and one gorilla died at the periphery of the Dja Reserve, Cameroon (32). All great apes were positive for the B. anthracis-specific pag and capC virulence genes. MLVA analyses showed that two different but related strains of B. anthracis had infected the great apes from Côte d'Ivoire and Cameroon, respectively. Interestingly, these strains form a highly distinct cluster separate from all other previously described B. anthracis strains, and further genetic analyses showed that these strains are significantly different from “classic” B. anthracis strains. They lack the four B. anthracis-specific prophage regions, regions A, C, D, and E (46), and new alleles of the two toxin genes pag and cya were identified (34).
In this study, we present data on the isolation and microbiological differentiation of bacteria from chimpanzees and one gorilla. Our results indicate that the isolates from Côte d'Ivoire (termed B. anthracis CI) and Cameroon (termed B. anthracis CA) are almost identical, with virulence plasmids closely related to those of B. anthracis in a chromosomal background of a new member of the B. cereus group.
MATERIALS AND METHODS
Animal samples and bacterial strains.
Six organ samples collected from three chimpanzees from Côte d'Ivoire stored at <−70°C were investigated (31). The samples were collected from intact carcasses of two animals (Léo and Olduvai) and from the partially opened carcass of one animal (Dorry). For the Cameroon apes, a cranium and bones were stored at room temperature, and a muscle sample was stored in RNAlater reagent (QIAGEN, Hilden, Germany) at room temperature. Samples from the muscle and the bone marrow of one chimpanzee and the tooth of a gorilla were studied (Table 1). All isolations were performed under biosafety level 3 conditions. Other bacterial strains from the B. cereus group used for MLST were B. cereus NCCB 72001 (same as ATCC 10987), B. cereus DSM 31 (same as ATCC 14579), B. cereus DSM 4312, B. cereus DSM 2301, B. cereus DSM 609, B. cereus DSM 4490 (same as ATCC 11778), B. thuringiensis DSM 350, B. thuringiensis DSM 2046, B. thuringiensis DSM 5815, B. mycoides DSM 2048, and B. weihenstephanensis DSM 11821. These strains were purchased from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (DSMZ) (Braunschweig, Germany) and The Netherlands Culture Collection of Bacteria (Utrecht, The Netherlands) as indicated. The 17 classic B. anthracis strains were described recently (26). The sequences of B. cereus E33L (formerly termed “Zebra Killer,” [ZK]; GenBank accession number NC_006274), B. cereus G9241 (accession number NZ_AAEK00000000), B. cereus ATCC 4342 (17, 45), and B. thuringiensis serovar konkukian strain 97-27 (accession number NC_005957) were derived from the corresponding databases.
TABLE 1.
Results of bacteriological examinations of great apes from Côte d'Ivoire and Cameroon
| Ape | Resultd
|
|||
|---|---|---|---|---|
| Clinical sample | Direct culture | Direct culture, 65°C | Enrichment culture, 65°C | |
| Chimpanzee (Léo)a | Spleenc | +++ | − | − |
| Lungc | +++ | − | − | |
| Chimpanzee (Olduvai)a | Lung | +++ | − | − |
| Liverc | +++ | − | − | |
| Chimpanzee (Dorry)a | Spleen | +++ | − | − |
| Lungc | + | + (2 colonies) | + | |
| Chimpanzeeb | Bone Marrowc | + (8 colonies) | ND | + |
| Musclec | + (1 colony) | ND | ND | |
| Gorillab | Toothc | + (1 colony) | ND | ND |
From Côte d'Ivoire.
From Cameroon.
DNA isolation from B. anthracis colonies derived from these samples.
−, no suspicious colonies detected; +, few suspicious colonies detected; +++, high incidence of suspicious colonies. ND, not done.
Microbiological studies.
After the addition of sterile distilled water, the organ samples from the chimpanzees from Côte d'Ivoire were homogenized using a mortar. One aliquot was heated at 65°C for 30 min to kill vegetative cells. The untreated and the heat-treated aliquots were plated onto the following agar plates and designated as direct cultures: Columbia agar (Oxoid, Wesel, Germany) with 5% sheep blood, Luria-Bertani (LB) agar, blood-trimethoprim agar (with anthrax supplement [1.6 mg trimethoprim, 6.4 mg sulfamethoxazole, and 20 mg polymyxin B per liter agar medium]), PLET agar (40 g Difco heart infusion agar, 30,000 units polymyxin B, 40 mg lysozyme, 200 mg EDTA, and 40 mg thallous acetate per liter agar medium), Cereus Ident agar (Heipha Diagnostica, Eppelheim, Germany) with the chromogenic substrate 5-bromo-4-chloro-3-indoxyl-myoinositol-1-phosphate (44), and Cereus selective agar (Merck, Darmstadt, Germany). In addition, 10 ml of liquid enrichment medium (LB or LB with anthrax supplement) was inoculated with either 0.1 ml of the nontreated or 0.1 ml of the heat-treated aliquot. As only very little material was available from the Cameroon apes, the bone marrow and muscle samples were homogenized as described above, but only the bone marrow sample was heated, and both the heat-treated and the nontreated aliquots were cultivated in enrichment medium. The tooth was incubated in liquid LB medium for a few minutes, and the medium was subsequently streaked onto the different agar plates.
After 24 h of incubation at 37°C, all enrichment cultures were streaked onto the plates indicated above for isolation. The presence of B. anthracis in enrichment cultures was tested by real-time PCR as described below. Colony growth was monitored daily. If a high number of colonies suspicious for B. anthracis was observed on plates with direct cultures, the corresponding enrichment cultures were not differentiated further. Suspicious colonies were subcultured on the solid media described above.
Tests for susceptibility to penicillin G and the gamma phage test were performed as described in the WHO Manual for Laboratory Diagnosis of Anthrax (57). Results of the gamma phage assay were read after 6 to 8 h and after 24 h of incubation at 37°C. Motility of bacteria was observed microscopically by hanging-drop preparation (WHO manual) and by observing growth in tubes with API M motility medium (BioMérieux, Nürtingen, Germany). Gram staining was performed using the microscopy Gram color reagents from Merck. Further bacteriological examinations were performed for selected isolates. Formation of the capsule was tested by cultivation on bicarbonate agar under a 5% CO2 atmosphere. Sensitivity to different antibiotics was analyzed using the Etest (VIVA Diagnostika, Cologne, Germany). The biochemical capacity was tested using the API 50 CHB system (BioMérieux).
Electron microscopy.
At least one bacterial isolate from each great ape was studied by electron microscopy. As a control, the classic B. anthracis isolate UDIII-7 was used. All bacterial samples (agar or suspension cultures) were first fixed at a biosafety level 3 laboratory in 10% formaldehyde including 1% glutaraldehyde in 0.05 M HEPES buffer (pH 7.2) for at least 2 h. The agar cultures were gently washed with distilled water prior to fixation with 1% OsO4 for scanning electron microscopy (SEM). After stepwise dehydration in graded alcohol, the samples were critical point dried in CO2 (CPD 030; BAL TEC, Vaduz, Liechtenstein), mounted onto the sample stubs, sputter coated with 3 nm Au/Pd (Polaron Sputter Coating Unit E 5100; GaLa Instrumente, Bad Schwalbach, Germany), and examined with a LEO FEG-1530 scanning electron microscope (Carl Zeiss SMT AG, Oberkochen, Germany) at 5 kV.
After a short wash with distilled water, the fixed suspension cultures were first agar block embedded by mixing equal volumes of concentrated bacteria and low-melting-point agar (3% phosphate-buffered saline) and postfixed with OsO4. After block staining with uranyl acetate (2% in distilled water), the samples were dehydrated stepwise in graded alcohol and embedded in LR-White (Science Service, Munich, Germany), which was polymerized at 60°C overnight. The ultrathin sections for transmission electron microscopy (TEM) were prepared with an ultramicrotome (Ultracut S; Leica, Wetzlar, Germany) and placed onto naked 400-mesh grids or onto Pioloform-F (Wacker Chemie, Munich, Germany)-coated 100-mesh grids. The sections were stained with lead citrate and stabilized with carbon evaporation (BAE 250; BAL TEC). The sections were examined using a TEM 902 (Carl Zeiss SMT AG) at 80 kV, and the images were digitized using a slow-scan charge-coupled-device camera (Pro Scan; Scheuring, Germany).
Concentrated bacteria were adsorbed for 1 min on the Pioloform-F-coated, carbon-stabilized, and glow-discharged copper grids and washed three times on droplets of distilled water. After negative staining with 1% uranyl acetate (pH 4 to 4.5), the sample was analyzed by TEM.
Molecular methods.
Bacterial DNA was isolated according to the protocol for gram-positive bacteria of the DNeasy tissue kit (QIAGEN). For inactivation, colony material of characterized strains of B. anthracis, as well as material from B. anthracis strains CI and CA, was autoclaved (121°C, 20 min) before DNA isolation.
Real-time PCR was performed with 50-μl volumes using either 1 μl of purified DNA, 3 μl of bacterial culture, or spiked colonies, as described previously by Ellerbrok et al. (14). Conventional PCR for the detection of the Ba813 fragment was performed according to a method described previously by Patra et al. (43). The fragment was sequenced using the ABI PRISM FS BigDye Terminator Cycle Sequencing Ready Reaction kit and an ABI PRISM 3100 genetic analyzer (Applied Biosystems, Darmstadt, Germany). Sequence data were analyzed with the LaserGene software. PCRs for amplification of the SG-749 fragment were performed as described previously (9). Ten microliters of the SG-749 PCR products was restricted with 10 units of AluI (MBI Fermentas, St. Leon-Rot, Germany) and analyzed by agarose gel electrophoresis. In addition, the PCR products were sequenced. MLST was performed by applying the schemes described previously by Helgason et al. (17) and Priest et al. (45). Sequencing was performed as described above. Other conventional PCR and sequencing analyses were performed according to standard procedures using Taq polymerase (MBI Fermentas) and the ABI PRISM FS BigDye Terminator Cycle Sequencing Ready Reaction kit.
Plasmids of B. anthracis strains were isolated from exponentially growing cultures according to a method described previously by Jensen et al. (23) and separated on a 0.7% agarose gel. Southern analysis was performed by capillary transfer (52), and hybridization with digoxigenin-labeled probes was carried out at 50°C. The same fragments of the capC and pag genes used for real-time PCR (14) were labeled with PCR DIG labeling mix (Roche, Mannheim, Germany), and the blot was developed using anti-digoxigenin antibodies conjugated to alkaline phosphatase and CDP Star according to the manufacturer's protocol (Roche).
For the detection of protective antigen (PA), bacteria were grown to late log phase in LB broth buffered with 100 mM HEPES (pH 8.0) and 0.8% (wt/vol) sodium bicarbonate in an atmosphere containing 5% CO2 at 37°C. Culture supernatants were analyzed by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis using standard procedures and subsequently transferred onto an Immobilon P polyvinylidene difluoride membrane (Millipore, Schwalbach, Germany) by semidry blotting. Western blot analysis was performed essentially as described previously (51) by using a monoclonal anti-PA antibody (clone 138; Senova, Jena, Germany) in a concentration of 1.5 μg/ml and a goat anti-mouse antibody conjugated to horseradish peroxidase (Dianova, Hamburg, Germany) in a concentration of 1 μg/ml. Signals were visualized on X-ray film (Hyperfilm ECL; Amersham Biosciences, Freiburg, Germany) by chemiluminescence (ECL substrate; Pierce, Bonn, Germany). As a positive control, 20 ng of a purified recombinant PA preparation kindly provided by W. Beyer, Hohenheim, was used.
Phylogenetic analysis (neighbor-joining tree) was performed using the Phylip algorithm.
RESULTS
Microbiological and electron microscopical characterization.
Colonies were assessed to be suspicious for B. anthracis if they showed the following features: possession of a so-called “Medusa head” (curl-like projections from the colony edge), no phospholipase C activity (no color change on Cereus Ident agar), no fermentation of mannitol and weak lecithinase activity (pink color and faint precipitation on Cereus selective agar), and distinct growth on blood trimethoprim and on PLET agar. Colonies or suspension cultures were screened for the presence of plasmids as described previously (14). Suspicious colonies could be isolated directly from samples of all five apes examined. However, as indicated in Table 1, the incidence of suspicious colonies differed among the samples, being very high in five of six organ samples from chimpanzees of Côte d'Ivoire (CI) that had been stored at −70°C and being very low (two times one and eight colonies, respectively) in the three samples from apes from Cameroon (CA). The latter samples contained a large number of nonanthrax Bacillus species, most likely due to contamination with the soil from the rainforest. After heating of the samples at 65°C, suspicious colonies were detected only in direct and enrichment cultures of one lung sample from Côte d'Ivoire and in the enrichment culture of the bone marrow sample from Cameroon (Table 1). This indicates that the bacteria had already formed spores in these two samples, whereas only vegetative cells were present in the organ samples from intact carcasses.
After 24 h of incubation at 37°C, suspicious colonies from direct or enrichment cultures (primary cultures) of all ape samples were similar and showed the suspicious features described above. On Columbia blood agar, colonies were approximately 5 mm in diameter, rough, and gray-greenish, and some had a mucoid center. The colonies grew with “Medusa head” like classic B. anthracis colonies (Fig. 1A to D) and were not beta-hemolytic. After 48 h of incubation, however, growth of the same colonies was atypical for B. anthracis. The colonies had a diameter of more than 10 mm with a smooth, shiny, mucoid, yellow-greenish center (diameter, >5 mm) and a dry, gray, weakly jagged edge. Growth on PLET agar was more inhibited than usual for B. anthracis, and CI and CA colonies were substantially smaller than typical B. anthracis colonies after a 2-day incubation.
FIG. 1.
Colony morphology and capsule production studied by SEM and TEM. Bacteria isolated from great apes (A, C, E, and G) and classic B. anthracis strains (B, D, F, and H) presented the same morphological criteria. The same colony morphology on agar was seen by SEM (A to D). Cells from suspension cultures showed the same capsule and filament (piles) morphology by negative staining (E and F), and a capsule fringe at the outer wall of the bacteria in thin cross-sections by TEM (G and H). Similar results were obtained with all bacterial isolates tested.
The essential characteristics of the B. anthracis isolates CI and CA were compared with those of classic B. anthracis strains and nonanthrax strains of the B. cereus group (Table 2). In contrast to classic B. anthracis strains, all isolates from primary cultures from apes were resistant to the gamma phage and motile. In hanging-drop preparations, even short chains of bacteria were clearly motile in some cases. Broth cultures and semisolid motility media were evenly turbid. However, no inverted fir tree effect was observed in tubes with motility medium (data not shown).
TABLE 2.
Bacteriological discrimination characteristics of atypical B. anthracis strains isolated from great apes, classic B. anthracis strains, and other strains of the B. cereus group
| Microbiological characteristic | Resulta
|
|||||
|---|---|---|---|---|---|---|
|
B. anthracis CI
|
B. anthracis CA
|
B. anthracis | B. cereus | |||
| Primary culture | Subculture | Primary culture | Subculture | |||
| Hemolysis | − | +/− | − | +/− | − | + |
| Motility | + | + | + | + | − | + |
| Susceptibility to gamma phage | − | +/− | − | +/− | + | − |
| Penicillin G | S | S/R | R | R | S | R |
| Capsule | +b | +/− | + | +/− | + | Absent in vitroc |
S, sensitive; R, resistant; −, negative; +, positive; +/−, some subclones positive, others negative.
Capsule production on bicarbonate agar under a CO2 atmosphere and on blood agar under an ambient atmosphere.
Certain other Bacillus spp. can produce a polypeptide capsule but not under normal culture conditions.
Negative staining electron microscopy revealed peritrich flagellation of the B. anthracis CI and CA strains, but no flagella were seen on cells of a classic B. anthracis strain (Fig. 2A and B). Gram staining of CI and CA isolates showed gram-positive rods and chains that differed in thickness. Spores that did not cause swelling of the cell were formed (data not shown). The difference in cell thickness was also confirmed by scanning electron microscopical studies that revealed capsule-like surface structures on a fraction of the cells (Fig. 2C). These structures were not seen on the surface of cells from a classic B. anthracis strain (Fig. 2D). In addition, twisted forms of the bacilli were frequently observed for CI and CA strains and rarely observed for a classic B. anthracis strain (Fig. 2E and F), although these atypical cell morphologies were described previously (29).
FIG. 2.
Cell morphology studied by TEM and SEM. Bacteria isolated from great apes (A, C, and E) and classic B. anthracis strains (B, D, and F) presented different morphological criteria. Cells from suspension cultures studied by TEM had flagella (A), in contrast to what was seen in B. Cells from agar cultures studied by SEM showed capsule structures (arrows) on the bacteria, in C in contrast to D, and twisted bacteria (E) in contrast to very rare structures (arrows) shown in F. Similar results were obtained with all bacterial isolates tested. All bars represent 1 μm.
As expected, a capsule was detected after cultivation of a classic B. anthracis strain and the CI and CA strains on bicarbonate agar in a CO2-enriched atmosphere (Fig. 1E to H). Surprisingly, the cells were also encapsulated when the CI and CA strains were grown on common media like Columbia blood or LB agar under ambient atmosphere. This capsule accounts for the structures observed on the surface of bacterial cells and for the mucoid appearance of the colonies.
Susceptibility to penicillin G varied for the B. anthracis CI and CA strains (Table 2). Whereas the CI isolates were sensitive, isolates from Cameroon were resistant to penicillin G. However, resistant colonies were also found in subclones of the CI isolate. Susceptibility to a panel of antibiotics was determined using the Etest (Table 3). The MICs determined for the different strains and isolates were comparable for most antibiotics except for tetracycline and amoxicillin-clavulanic acid, in which case the CA strain was less susceptible. For the latter antibiotic, some CA isolates showed intermediate sensitivity. All other MICs indicated sensitivity of the B. anthracis CI and CA strains to all antibiotics used in the Etest.
TABLE 3.
Etest results for atypical B. anthracis isolates
| Antibiotic | MIC range (μg/ml)a
|
|
|---|---|---|
| B. anthracis CI | B. anthracis CA | |
| Amoxicillin-clavulanic acidb | 0.016-0.023 | 3.0-6.0c |
| Ciprofloxacin | 0.038 | 0.047-0.5 |
| Doxycycline | 0.016 | 0.064-0.25 |
| Clindamycin | 0.5-0.75 | 0.75-1.0 |
| Imipenem | 0.047-0.064 | 0.064-0.94 |
| Piperacillin | 0.75-1.5 | 0.75-3.0 |
| Rifampin | 0.19-0.25 | 0.125-0.5 |
| Tetracycline | 0.032-0.47 | 0.25-1.5 |
| Vancomycin | 1.5-3.0 | 2.0-3.0 |
Several isolates were tested for each antibiotic.
Sensitive, MIC ≤ 4 μg/ml; resistant, MIC ≥ 8 μg/ml.
Some isolates showed intermediate sensitivity.
Analysis of the biochemical properties of the CI isolates using the API 50 CHB system showed positive reactions for d-ribose, d-glucose, N-acetylglucosamine, arbutin, esculin ferric citrate, salicin, d-cellobiose, d-maltose, d-saccharose, d-trehalose, AmiDon (starch), glycogen, potassium gluconate, gelatin, and nitrate. For the Voges-Proskauer reaction and gluconate or nitrate metabolism, differences between single isolates were observed. CA isolates were positive for d-fructose and nitrate and negative for gluconate and the Voges-Proskauer reaction. All other reactions were identical to those from the CI isolates. Single isolates differed in the metabolism for d-trehalose and gelatin. These reaction patterns point with a high probability towards the presence of B. anthracis, but a definitive diagnosis is not possible with these biochemical criteria.
Some bacterioscopic findings were not typical for B. anthracis. Due to the different sizes of the bacterial rods and chains (Fig. 2C) and the partially twisted forms (Fig. 2E), the presence of mixed cultures was assumed. To exclude mixed cultures, single colonies were repeatedly subcultured on different agar plates and retested (Table 2). In these subclones, colonies with very different morphologies and characteristics were observed. For example, small smooth colonies were found, which, in contrast to the primary cultures, exhibited strong beta-hemolysis and were sensitive to the gamma phage. All colonies remained negative for phospholipase C activity. Real-time PCR assays revealed the presence of the pag gene in all subclones, confirming that all of these subcloned isolates are B. anthracis-like. The ability to form a capsule was also variable in the subclones, and could be correlated with the loss of the capC marker as indicated by real-time PCR.
Molecular characterization.
Real-time PCR assays targeting the plasmid markers pag (on plasmid pXO1) and capC (on plasmid pXO2) as well as the chromosomal marker rpoB were performed with initially isolated suspicious colonies, with enrichment cultures, and with DNA preparations (14). Both primary cultures and subcultures of B. anthracis strains CI and CA were analyzed. In all cases, fluorescence signals of the pag marker appeared early, with cycle threshold (CT) values of 20 to 25, whereas signals of the rpoB target were delayed with CT values above 30. The capC gene marker was positive in PCR assays of primary cultures (CT values of 20 to 25) and in the majority of the subclones. The occurrence of delayed signals of the rpoB marker had been observed previously for some strains of the B. cereus group (14, 26) and was explained by residual nonspecific priming of the B. anthracis-specific primers on the closely related B. cereus genomic sequences.
Further analyses were performed with purified DNA from B. anthracis CI and CA that was isolated from primary cultures derived from different organ samples (Table 1). The results described below were the same for all isolates tested. The Ba813 gene fragment was amplified from all DNA preparations and sequenced. Compared to the Ba813 sequence found in classic B. anthracis strains, the fragment possessed two nucleotide differences (data not shown).
AluI restriction of the SG-749 fragment, a randomly amplified polymorphic DNA marker specific for the B. cereus complex, revealed a unique restriction type for all classic B. anthracis strains tested (9, 26). However, in B. anthracis strains CI and CA, a different restriction pattern was found, which was identical to the patterns found in the environmental isolates B. cereus Hohenheim, Bacillus sp. strain 2617, and Bacillus sp. strain 153 (26). Sequencing of the SG-749 fragment of B. anthracis strains CI and CA revealed six nucleotide differences compared to classic B. anthracis strains (data not shown), confirming the restriction pattern through the identification of an additional AluI restriction site, which results in restriction fragments of 496 bp, 166 bp, and 89 bp compared to fragments of 662 bp and 89 bp for classic B. anthracis strains.
MLST was performed to assess the phylogenetic relationship of the B. anthracis CI and CA strains with classic B. anthracis strains and other strains of the B. cereus group. Two recently described typing schemes, both based on fragments of seven housekeeping genes, were applied (17, 45). The sequences of all 14 gene fragments were identical for different isolates of B. anthracis strains CI and CA. The results are summarized in Table 4. According to the typing scheme described previously by Helgason et al. (17), none of the seven alleles was identical to those found in classic B. anthracis strains like the UDIII-7 strain. The alleles from B. anthracis strains CI and CA and B. anthracis strain UDIII-7 differed by one to three nucleotides. According to the typing scheme described previously by Priest et al. (45), the gmk and pta alleles of strains CI and CA were identical to the alleles from B. anthracis strain UDIII-7; the other five alleles differed by between 2 and 19 nucleotides. The alleles for glpT, pyrE, sucC, and ilvD had not been observed previously. Most classic B. anthracis strains in our strain collection had sequence type 1 (ST-1) according to both typing schemes. Only strain 5261 had ST-2 (Fig. 3), and strain B19-39 possessed a new pyrE allele, which differed by one nucleotide from allele 19 and which was not identical to the corresponding allele from B. anthracis strains CI and CA, where two nucleotide differences at other positions were observed (Table 4).
TABLE 4.
Results of multilocus sequence typing
| Allele | Allele no. found in:a
|
No. of nt differencesb | Highest homology in BLAST search | |
|---|---|---|---|---|
| B. anthracis strains CI and CA | B. anthracis strain UDIII-7 | |||
| adkc | 2 | 25 | 1 | 100%, B. thuringiensis serovar konkukian |
| ccpAc | 35 | 36 | 1 | 100%, B. thuringiensis serovar konkukian |
| ftsAc | 8 | 2 | 2 | 100%, B. thuringiensis serovar konkukian |
| glpTc | (18) | 18 | 1 | 99%, B. anthracis Ames |
| pyrEc | (19) | 19 | 2 | 99%, B. anthracis Ames |
| recFc | 11 | 9 | 1 | 100%, B. thuringiensis serovar konkukian |
| sucCc | (1) | 12 | 3 | 99%, B. cereus E33L |
| glpFd | 34 | 1 | 6 | 98%, B. thuringiensis serovar konkukian |
| gmkd | 1 | 1 | 0 | 100%, B. anthracis Ames |
| ilvDd | (51, 56, 57) | 1 | 6 | 99%, B. thuringiensis serovar konkukian |
| ptad | 1 | 1 | 0 | 100%, B. anthracis Ames |
| purd | 18 | 1 | 19 | 99%, B. thuringiensis serovar konkukian |
| pycAd | 29 | 1 | 2 | 99%, B. thuringiensis serovar konkukian |
| tpid | 5 | 1 | 5 | 99%, B. cereus E33L |
For new alleles, the number(s) of alleles with highest homology is given in parentheses.
Number of nucleotide (nt) differences between alleles of B. anthracis CI or CA and B. anthracis UDIII-7.
Alleles belong to the scheme described previously by Helgason et al. (17).
Alleles belong to the scheme described previously by Priest et al. (45).
FIG. 3.
Neighbor-joining phylogenetic trees for the concatenated allele sequences of different strains of the B. cereus (Bc) group. (A) Tree based on the scheme described previously by Helgason et al. (17) comparing 2,977 bp. B. anthracis strain B19-39 possessed a new pyrE allele, and the other 16 classic B. anthracis strains had ST-1. (B) Tree based on the scheme described previously by Priest et al. (45) comparing 2,838 bp. B. anthracis strain 5261 was ST-2, and the other 16 B. anthracis strains were ST-1. Other STs that could be assigned according to the database at http://pubmlst.org/bcereus/ were as follows: ST-4 (B. cereus ATCC 14579), ST-32 (B. cereus ATCC 10987), ST-26 (B. cereus DSM 4312), ST-34 (B. cereus ATCC 11778), ST-10 (B. thuringiensis [Bt] DSM 2046 and DSM 350), ST-16 (B. thuringiensis DSM 5815), ST-116 (B. mycoides [Bm] DSM 2048), ST-38 (B. cereus ATCC 4342), and ST-113 (B. thuringiensis serovar konkukian strain 97-27). The trees were statistically evaluated with a bootstrap analysis with 1,000 bootstraps. Only relevant bootstrap values above 70% are shown.
A BLAST search revealed the highest homology for the alleles from B. anthracis CI and CA with the corresponding alleles from classic B. anthracis strains, from B. thuringiensis serovar konkukian strain 97-27, and from B. cereus strain E33L. To analyze the phylogenetic relationship of B. anthracis CI and CA to classic B. anthracis strains and to other Bacillus strains, the allele sequences of each typing scheme were concatenated. The concatenated sequences had a length of approximately 3,000 bp for each of the typing schemes. The number of nucleotide differences between B. anthracis UDIII-7 and the B. anthracis CI or CA strain was 11 for the scheme described previously by Helgason et al. (17) and 38 for the scheme described previously by Priest et al. (45). Based on the concatenated sequences, neighbor-joining phylogenetic trees were constructed (Fig. 3). Both trees confirmed the close relationship between B. anthracis CI and CA, classic B. anthracis strains, B. thuringiensis serovar konkukian strain 97-27, and B. cereus strain E33L. The sequence types (45) of the examined strains, if known, are indicated in the legend of Fig. 3.
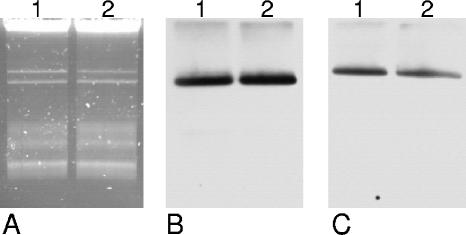
Virulent strains of B. anthracis possess two large virulence plasmids (27), the toxin plasmid pXO1 (182 kb), and the capsule plasmid pXO2 (95 kb). To determine whether the capC and pag genes of B. anthracis strain CI that were detected by real-time PCR were located on plasmids as well, the large plasmids were purified and separated by agarose gel electrophoresis. Southern analysis was performed with probes for the capC and pag genes. B. anthracis strain UDIII-7 was used as a control (Fig. 4). Each of the two plasmids was the same size in both strains, and in Southern analysis, the larger plasmid hybridized with the pag probe and the smaller plasmid hybridized with the capC probe. Therefore, we conclude the presence of two virulence plasmids related to those of classic B. anthracis strains.
FIG. 4.
Detection of large plasmids in B. anthracis isolates. Plasmids of B. anthracis strains CI (lane 1) and UDIII-7 (lane 2) were detected by agarose gel electrophoresis (A) or by Southern blot analysis with probes for the capC gene (B) or the pag gene (C).
The presence of several additional plasmid genes encoding unknown functions or virulence factors was assessed by PCR or by analysis of the unfinished plasmid sequences of the B. anthracis CI strain (sequencing is in progress) (H. Liesegang, personal communication). Thus, we confirmed the presence of the transcriptional regulator gene atxA, the toxin genes pag, lef, cya, and other genes on the larger plasmid (pXO1-04, pXO1-16, pXO1-45, pXO1-59, pXO1-65, pXO1-78, pXO1-87, pXO1-103, and pXO1-142, according to the sequence reported under GenBank accession number NC_001496) and the presence of the transcriptional regulator genes acpA and acpB, the capsule biosynthesis genes capA, capB, capC, and capD, and other genes on the smaller plasmid (pXO2-04, pXO2-16, pXO2-25, pXO2-28, pXO2-37, pXO2-38, pXO2-47, pXO2-66, pXO2-69, pXO2-81, and pXO2-84, according to the sequence reported under GenBank accession number NC_002146). The deduced protein sequences of the transcriptional regulators, the toxins, and the capsule biosynthesis enzymes contained no or only very few amino acid exchanges compared to their homologues in classic B. anthracis strains (data not shown).
Western blot analysis was performed to assess the expression of protective antigen by culture supernatants of B. anthracis strains CI and UDIII-7 grown in bicarbonate medium under a CO2 atmosphere (Fig. 5). The strains expressed proteins of the appropriate size (83 kDa) that reacted with a monoclonal antibody, confirming the secretion of PA by both B. anthracis cultures.
FIG. 5.
Western blot analysis demonstrating the expression of protective antigen. Culture supernatants of B. anthracis strains CI (lane 2) and UDIII-7 (lane 3) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane, and the protein was detected with a monoclonal anti-PA antibody. Purified recombinant PA was included as a control (lane 1). The position of the 85-kDa band of the protein standard is indicated.
DISCUSSION
We were able to isolate B. anthracis-like bacteria from all ape necropsy samples that tested positive by PCR for B. anthracis. The microbiological features, however, were uncommon because the bacteria were motile and resistant to the gamma phage, and some isolates were also resistant to penicillin G. These microbiological findings would exclude the presence of B. anthracis according to currently used differential diagnostic criteria (57). Motile strains were observed only rarely. Liang and Yu (36) previously described numerous motile strains from China with polar flagellation, in contrast to our isolates, which showed peritrich flagellation. Resistance to the gamma phage was seen in 15% of strains tested in a previous study (6). Naturally occurring penicillin G-resistant B. anthracis isolates have been reported previously (5, 30), and a survey of strains derived from carcasses and soil in South Africa has revealed penicillin G resistance in up to 16% of isolates (40). The fact that one diagnostic trait of an isolate does not meet the typical anthrax criteria would probably not compromise its correct identification. However, in primary cultures of the CI and CA strains, two or three features, respectively, argue against an identification as B. anthracis, namely, motility, resistance to the gamma phage, and resistance to penicillin G. Therefore, these isolates would probably be misdiagnosed in routine laboratories if diagnosis is based only on microbiological criteria. Also, testing of the biochemical properties using the API 50 CHB system turned out to be inapplicable for our isolates, because slight discrepancies in the reaction patterns resulted in differing identifications. Although the presence of B. anthracis was indicated with the highest probability, no definitive identification was possible. Antibiotic susceptibilities examined by Etest showed MICs in the same range as those described previously by Turnbull et al. (55) for isolates of the B. cereus group. However, for amoxicillin-clavulanic acid, ciprofloxacin, and tetracycline, MICs for B. anthracis isolates from Cameroon were clearly higher than those described for B. anthracis (MICs according to those reported previously by Turnbull et al. are as follows: amoxicillin-clavulanic acid, 0.016 to 0.5 μg/ml; ciprofloxacin, 0.032 to 0.094 μg/ml; tetracycline, 0.016 to 0.094 μg/ml [55]).
As confirmed by Western blot analysis, protective antigen was expressed by the B. anthracis CI strain and secreted into the medium when the culture was grown under bicarbonate/CO2 conditions. However, unlike classic B. anthracis strains, the CI and CA isolates formed a capsule when cultivated not only on bicarbonate agar in a CO2-enriched atmosphere but also on common agar medium under normal growth conditions. We do not yet know whether the strains express the anthrax-typical poly-γ-d-glutamic acid capsule, but the capsule biosynthesis genes are present and probably functional as suggested by sequencing data (not shown). Subculturing occasionally resulted in a loss of the capC marker and consequently in a loss of capsule production. The corresponding isolates will be further analyzed for the presence of the capsule plasmid, because it is known that pXO2 is easily and spontaneously lost (54). In some subclones, beta-hemolysis, sensitivity to the gamma phage, and resistance to penicillin G in previously sensitive CI isolates were observed. The reason for these variations is unclear. One could speculate that regulatory mechanisms that are functional in classic B. anthracis strains do not apply or are different in the new isolates. In classic B. anthracis strains, the expression of toxin and capsule genes as well as numerous other genes on both plasmids and the chromosome is controlled by the pXO1-encoded pleiotropic regulator AtxA (4, 28, 56). Capsule formation is affected by AtxA via positive control of two pXO2-encoded genes, acpA and acpB, which are responsible for the CO2-dependent expression of the capsule genes. Low levels of capB transcripts were detected during aerobic growth (13), which seemed to be initiated from a promoter not controlled by AcpA or AcpB. This low level of transcription, however, was not high enough to produce a detectable capsule on the cell surface (13). As the B. anthracis CI and CA strains form capsules under normal growth conditions, the regulation of capsule generation in these strains differs from that in classic anthrax strains. The reason for these expression differences can not yet be explained, because the atxA, acpA, and acpB genes encoding the transcriptional regulators are present on the pXO1- and pXO2-related plasmids of B. anthracis CI, and differences in regulation cannot be easily explained by a lack of one or more of these genes.
PlcR is a pleiotropic transcriptional regulator in nonanthrax strains of the B. cereus group that upregulates the expression of more than 100 genes, including those for hemolysis and motility, through binding to an upstream palindromic motif (15, 21, 35). Although these genes are present in classic B. anthracis strains, they are usually not expressed due to a nonsense mutation in the plcR gene. It was speculated that the acquisition of the pXO1 plasmid induced incompatibility of the regulator AtxA with the chromosomally encoded PlcR (38). Frameshift mutations in four essential genes of the flagellar gene cluster led to the loss of motility in B. anthracis (50). However, the data presented in this investigation indicate that flagellar genes are functional in B. anthracis strains CI and CA, and beta-hemolytic activity was observed in some subclones. Sequencing of the plcR gene of the B. anthracis CI strain revealed an insertion of 1 bp near the 3′ end of the gene but no nonsense mutation, resulting in a protein with a slightly altered C terminus and an extension of four amino acids compared to other PlcR proteins (data not shown). Therefore, it is possible that the new B. anthracis isolates possess a functional PlcR protein. The two genes for β-lactamases are usually not expressed in B. anthracis (7, 8). Their regulation is unclear, because they lack the upstream PlcR binding site, but variations in gene regulation might be the reason for penicillin G resistance in the B. anthracis CA isolates and in some CI subcultures.
Recently, a protein (GamR) involved in the bacterial receptor for the gamma phage was identified (10). Sequencing data indicate that a gene with homology to the gamR gene is present (data not shown), but the protein and/or other proteins involved in gamma phage propagation are probably not expressed in the primary isolates. The reason for the altered behavior of some subcultures is not known, and no data for the regulation of the phage receptor are available yet. The phenotypic “instability” observed in subclones of the CI and CA strains might result from a relatively recent acquisition of the B. anthracis virulence plasmids by a member of the B. cereus group. The balanced regulation of different plasmid- and chromosome-encoded genes that is observed in classic B. anthracis strains might be the result of a long evolutionary process not yet accomplished in these atypical isolates.
Based on microbiological criteria, the B. anthracis CI and CA isolates slightly differed by their antibiotic susceptibilities and biochemical capacities. Previous analyses also revealed different genotypes in MLVA and slightly different pag sequences. Phylogenetic tree analyses of the gyrB and the rpoB genes revealed close relations between the CI and CA strains. However, while gyrB clustered with classic B. anthracis isolates, the rpoB sequences were distinct from those of other B. anthracis isolates and, rather, showed homologies to other members of the B. cereus group (31, 34). To further characterize these isolates, molecular criteria that are currently used to distinguish B. anthracis from other isolates of the B. cereus group, like MLST, were applied. These molecular analyses underlined that B. anthracis strains CI and CA are closely related to each other and clearly showed that they differ from “classic” B. anthracis strains.
The presence of the Ba813 marker is not specific for B. anthracis strains, as was previously shown for several exceptions (26, 47). AluI restriction of the SG-749 fragment resulted in the same pattern for all classic B. anthracis strains tested, but B. anthracis strains CI and CA exhibited a pattern that was described previously for some other nonanthrax strains of the B. cereus group (26). The only classic B. anthracis feature was the presence of two large plasmids with sizes comparable to those of pXO1 and pXO2, possessing the pag and capC genes, respectively.
MLST confirmed the close relationship of strains CI and CA with classic B. anthracis strains but also with two virulent atypical members of the B. cereus group (Fig. 3). B. thuringiensis is known to be an insect pathogen, but B. thuringiensis serovar konkukian strain 97-27 was originally isolated from a case of severe human tissue necrosis and was pathogenic in immunosuppressed mice and thus, in this respect, rather resembled B. anthracis (18, 19). The strain possesses the 77-kb plasmid pBT9727 with many open reading frames homologous to sequences of the capsule plasmid pXO2, excluding the sequences necessary for capsule biosynthesis. The second closely related strain, B. cereus strain E33L, was originally isolated from the carcass of a dead zebra suspected to have died of anthrax in Namibia. It contains two large and three small plasmids, which do not encode homologues of known virulence factors in B. anthracis, B. cereus, or B. thuringiensis (48). Recently, a B. cereus strain was isolated from a patient with inhalation anthrax-like illness. This strain, termed G9241, possesses two plasmids, with one (pBCXO1, 191 kb) having 99.6% similarity to the B. anthracis toxin plasmid pXO1 (20). Gene products with similarities to pXO1-encoded gene products were also found on the 218-kb plasmid pBC218. This plasmid encodes gene products for a polysaccharide capsule cluster but not the gene products for the poly-γ-d-glutamic acid found in B. anthracis. Interestingly, B. cereus G9241 appears to encode fully functional copies of both PlcR and AtxA, and capsule production is not regulated by increased CO2 concentrations. In contrast to the two strains 97-27 and E33L, the relationship of B. cereus G9241 with B. anthracis is less pronounced when housekeeping genes are analyzed by MLST.
At present, we can only speculate how the atypical B. anthracis isolates CI and CA evolved. It can be hypothesized that these strains that were isolated in two regions more than 1,000 miles apart represent an old form that shares a common ancestor with classic B. anthracis strains. Another possibility would be a more recent emergence based on the transfer of the B. anthracis plasmids to an unknown strain of the B. cereus group. Although plasmid transfers were never documented in natural populations, it has been demonstrated that pXO1 and pXO2 could be transferred by conjugative plasmids originating in B. thuringiensis (2). Up to now, the CI and CA strains were observed only in rainforests, and their epidemiology is unknown. The source of infection of the great apes remains unclear. Long-term observation of the habituated chimpanzees in the Taï National Park showed that they not only feed on plants or arthropods but also hunt red colobus monkeys (3). However, anthrax infections have not been found in these prey monkeys or other mammals in the areas to date, whereas in general, disease surveillance in such remote regions is challenging and has not been performed systematically (33). It is known that blowflies that feed on infected carcasses are able to disseminate anthrax in their excretions (22). Leaves can be heavily contaminated with these infective excretions, but this source of infection remains speculative. Even contaminated water resources cannot be excluded.
We presented the microbiological and molecular analysis of very atypical isolates of B. anthracis, which would probably not have been detected by routine diagnostics. The isolates CI and CA possess virulence plasmids closely related to those of B. anthracis but a chromosomal background that is closer to those of atypical B. cereus and B. thuringiensis strains. In addition, gene regulation was different from that of classic B. anthracis strains, and the isolates behaved in an unstable manner upon subcultivation. This might be a hint that the cross talk between chromosome and plasmids is not yet balanced. It was previously suggested that major phenotypic differences between members of the B. cereus group might represent alterations in gene expression rather than sequence divergence (21, 49, 50). Sequence analysis of the whole genome of the B. anthracis CI isolate will give further information on the relationship of the isolates to other members of the B. cereus group. The first evidence for the virulence properties of the CI isolate was shown by its capsule and protective antigen expression, but the virulence of the strains will be further confirmed by in vitro and animal studies.
Acknowledgments
We thank the Ivorian authorities for long-term support, especially the Ministry of the Environment and Forests as well as the Ministry of Research, the directorship of the Taï National Park, the Laboratoire National d'Appui au Développement Agricole (LANADA) and Laboratoire Central de Pathologie Animale (LCPA), Bingerville, Côte d'Ivoire, and the Swiss Research Center in Abidjan. From Cameroon, we thank the MINFoF, MINRESI, the Service de la Conservation de la Réserve du Dja, and various organizations, especially Last Great Ape, the Limbe Wildlife Center, and Projet Grand Singe as well as the Cameroon Project, Department of Epidemiology, John Hopkins University Bloomberg School of Public Health. The field work was supported by the Max Planck Society.
For skillful technical support during necropsies, we thank T. Deschner, Y. Moebius, and S. Junglen. We are grateful to S. Becker, T. Franz, M. Urban-Schriefer, U. Buwitt, H. Emmel, and J. Tesch for expert technical laboratory assistance and to A. Jenzora for helping with Western blot analysis. We thank A. Rassbach and H. Böhnel for providing B. anthracis strains and W. Beyer for providing B. anthracis strains and purified protective antigen.
REFERENCES
- 1.Agaisse, H., M. Gominet, O. A. Økstad, N. Gilois, V. Sanchis, A. B. Kolstø, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. [DOI] [PubMed] [Google Scholar]
- 2.Battisti, L., B. D. Green, and C. B. Thorne. 1985. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J. Bacteriol. 162:543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boesch, C., and H. Boesch-Achermann. 2000. The chimpanzees of the Taï Forest: behavioural ecology and evolution. Oxford University Press, Oxford, United Kingdom.
- 4.Bourgogne, A., M. Drysdale, S. G. Hilsenbeck, S. N. Peterson, and T. M. Koehler. 2003. Global effects of virulence gene regulators in a Bacillus anthracis strain with both virulence plasmids. Infect. Immun. 71:2736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradaric, N., and V. Punda-Polic. 1992. Cutaneous anthrax due to penicillin-resistant Bacillus anthracis transmitted by an insect bite. Lancet 340:306-307. [DOI] [PubMed] [Google Scholar]
- 6.Buck, C. A., R. L. Anacker, F. S. Newman, and A. Eisenstark. 1963. Phage isolated from lysogenic Bacillus anthracis. J. Bacteriol. 85:1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y., J. Succi, F. C. Tenover, and T. M. Koehler. 2003. β-Lactamase genes of the penicillin-susceptible Bacillus anthracis Sterne strain. J. Bacteriol. 185:823-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y., F. C. Tenover, and T. M. Koehler. 2004. β-Lactamase gene expression in a penicillin-resistant Bacillus anthracis strain. Antimicrob. Agents Chemother. 48:4873-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daffonchio, D., S. Borin, G. Frova, R. Gallo, E. Mori, R. Fani, and C. Sorlini. 1999. A randomly amplified polymorphic DNA marker specific for the Bacillus cereus group is diagnostic for Bacillus anthracis. Appl. Environ. Microbiol. 65:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davison, S., E. Couture-Tosi, T. Candela, M. Mock, and A. Fouet. 2005. Identification of the Bacillus anthracis γ phage receptor. J. Bacteriol. 187:6742-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vos, V. 1994. Anthrax, p. 1262. In J. A. W. Coetzer, G. R. Thomson, R. C. Tustin, and N. P. J. Kriek (ed.), Infectious diseases of livestock. Oxford University Press, Oxford, United Kingdom.
- 12.Drobniewski, F. A. 1993. Bacillus cereus and related species. Clin. Microbiol. Rev. 6:324-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drysdale, M., A. Bourgogne, and T. M. Koehler. 2005. Transcriptional analysis of the Bacillus anthracis capsule regulators. J. Bacteriol. 187:5108-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellerbrok, H., H. Nattermann, M. Ozel, L. Beutin, B. Appel, and G. Pauli. 2002. Rapid and sensitive identification of pathogenic and apathogenic Bacillus anthracis by real-time PCR. FEMS Microbiol. Lett. 214:51-59. [DOI] [PubMed] [Google Scholar]
- 15.Gohar, M., O. A. Økstad, N. Gilois, V. Sanchis, A. B. Kolstø, and D. Lereclus. 2002. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2:784-791. [DOI] [PubMed] [Google Scholar]
- 16.Helgason, E., O. A. Økstad, D. A. Caugant, H. A. Johansen, A. Fouet, M. Mock, I. Hegna, and A. B. Kolstø. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helgason, E., N. J. Tourasse, R. Meisal, D. A. Caugant, and A. B. Kolstø. 2004. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl. Environ. Microbiol. 70:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez, E., F. Ramisse, J. P. Ducoureau, T. Cruel, and J. D. Cavallo. 1998. Bacillus thuringiensis subsp. konkukian (serotype H34) superinfection: case report and experimental evidence of pathogenicity in immunosuppressed mice. J. Clin. Microbiol. 36:2138-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, K. K., L. O. Ticknor, R. T. Okinaka, M. Asay, H. Blair, K. A. Bliss, M. Laker, P. E. Pardington, A. P. Richardson, M. Tonks, D. J. Beecher, J. D. Kemp, A. B. Kolstø, A. C. Wong, P. Keim, and P. J. Jackson. 2004. Fluorescent amplified fragment length polymorphism analysis of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis isolates. Appl. Environ. Microbiol. 70:1068-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmaster, A. R., J. Ravel, D. A. Rasko, G. D. Chapman, M. D. Chute, C. K. Marston, B. K. De, C. T. Sacchi, C. Fitzgerald, L. W. Mayer, M. C. Maiden, F. G. Priest, M. Barker, L. Jiang, R. Z. Cer, J. Rilstone, S. N. Peterson, R. S. Weyant, D. R. Galloway, T. D. Read, T. Popovic, and C. M. Fraser. 2004. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc. Natl. Acad. Sci. USA 101:8449-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, E. S. Dusko, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 22.Jensen, G. B., B. M. Hansen, J. Eilenberg, and J. Mahillon. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 5:631-640. [DOI] [PubMed] [Google Scholar]
- 23.Jensen, G. B., A. Wilcks, S. S. Petersen, J. Damgaard, J. A. Baum, and L. Andrup. 1995. The genetic basis of the aggregation system in Bacillus thuringiensis subsp. israelensis is located on the large conjugative plasmid pXO16. J. Bacteriol. 177:2914-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keim, P., A. Kalif, J. Schupp, K. Hill, S. E. Travis, K. Richmond, D. M. Adair, M. Hugh-Jones, C. R. Kuske, and P. Jackson. 1997. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J. Bacteriol. 179:818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keim, P., L. B. Price, A. M. Klevytska, K. L. Smith, J. M. Schupp, R. Okinaka, P. J. Jackson, and M. E. Hugh-Jones. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182:2928-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klee, S. R., H. Nattermann, S. Becker, M. Urban-Schriefer, T. Franz, D. Jacob, and B. Appel. 2006. Evaluation of different methods to discriminate Bacillus anthracis from other bacteria of the Bacillus cereus group. J. Appl. Microbiol. 100:673-681. [DOI] [PubMed] [Google Scholar]
- 27.Koehler, T. M. 2002. Bacillus anthracis genetics and virulence gene regulation. Curr. Top. Microbiol. Immunol. 271:143-164. [DOI] [PubMed] [Google Scholar]
- 28.Koehler, T. M., Z. Dai, and M. Kaufman-Yarbray. 1994. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J. Bacteriol. 176:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolle, W., R. Kraus, and P. Uhlenhuth. 1931. Handbuch der pathogenen Mikroorganismen. Gustav Fischer und Urban & Schwarzenberg, Jena, Germany.
- 30.Lalitha, M. K. and M. K. Thomas. 1997. Penicillin resistance in Bacillus anthracis. Lancet 349:1522. [DOI] [PubMed] [Google Scholar]
- 31.Leendertz, F. H., H. Ellerbrok, C. Boesch, E. Couacy-Hymann, K. Matz-Rensing, R. Hakenbeck, C. Bergmann, P. Abaza, S. Junglen, Y. Moebius, L. Vigilant, P. Formenty, and G. Pauli. 2004. Anthrax kills wild chimpanzees in a tropical rainforest. Nature 430:451-452. [DOI] [PubMed] [Google Scholar]
- 32.Leendertz, F. H., F. Lankester, P. Guislain, C. Néel, and O. Drori. Anthrax in western and central Africa great apes. Am. J. Primatol., in press. [DOI] [PubMed]
- 33.Leendertz, F. H., G. Pauli, K. Maetz-Rensing, W. Boardman, C. Nunn, H. Ellerbrok, S. A. Jensen, S. Junglen, and C. Boesch. Pathogens as drivers of population declines: the importance of systematic monitoring in great apes and other threatened mammals. Cons. Biol., in press.
- 34.Leendertz, F. H., S. Yumlu, G. Pauli, C. Boesch, E. Couacy-Hymann, L. Vigilant, S. Junglen, S. Schenk, and H. Ellerbrok. 2006. A new Bacillus anthracis found in wild chimpanzees and a gorilla from west and central Africa. PLoS Pathog. 2:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lereclus, D., H. Agaisse, M. Gominet, S. Salamitou, and V. Sanchis. 1996. Identification of a Bacillus thuringiensis gene that positively regulates transcription of the phosphatidylinositol-specific phospholipase C gene at the onset of the stationary phase. J. Bacteriol. 178:2749-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang, X., and D. Yu. 1999. Identification of Bacillus anthracis strains in China. J. Appl. Microbiol. 87:200-203. [DOI] [PubMed] [Google Scholar]
- 37.Makino, S., I. Uchida, N. Terakado, C. Sasakawa, and M. Yoshikawa. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mignot, T., M. Mock, D. Robichon, A. Landier, D. Lereclus, and A. Fouet. 2001. The incompatibility between the PlcR- and AtxA-controlled regulons may have selected a nonsense mutation in Bacillus anthracis. Mol. Microbiol. 42:1189-1198. [DOI] [PubMed] [Google Scholar]
- 39.Miller, J. M., J. G. Hair, M. Hebert, L. Hebert, F. J. Roberts, Jr., and R. S. Weyant. 1997. Fulminating bacteremia and pneumonia due to Bacillus cereus. J. Clin. Microbiol. 35:504-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odendaal, M. W., P. M. Pieterson, V. De Vos, and A. D. Botha. 1991. The antibiotic sensitivity patterns of Bacillus anthracis isolated from the Kruger National Park. Onderstepoort J. Vet. Res. 58:17-19. [PubMed] [Google Scholar]
- 41.Okinaka, R. T., K. Cloud, O. Hampton, A. R. Hoffmaster, K. K. Hill, P. Keim, T. M. Koehler, G. Lamke, S. Kumano, J. Mahillon, D. Manter, Y. Martinez, D. Ricke, R. Svensson, and P. J. Jackson. 1999. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J. Bacteriol. 181:6509-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Økstad, O. A., M. Gominet, B. Purnelle, M. Rose, D. Lereclus, and A. B. Kolstø. 1999. Sequence analysis of three Bacillus cereus loci carrying PIcR-regulated genes encoding degradative enzymes and enterotoxin. Microbiology 145:3129-3138. [DOI] [PubMed] [Google Scholar]
- 43.Patra, G., P. Sylvestre, V. Ramisse, J. Therasse, and J. L. Guesdon. 1996. Isolation of a specific chromosomic DNA sequence of Bacillus anthracis and its possible use in diagnosis. FEMS Immunol. Med. Microbiol. 15:223-231. [DOI] [PubMed] [Google Scholar]
- 44.Peng, H., V. Ford, E. W. Frampton, L. Restaino, L. A. Shelef, and H. Spitz. 2001. Isolation and enumeration of Bacillus cereus from foods on a novel chromogenic plating medium. Food Microbiol. 18:231-238. [Google Scholar]
- 45.Priest, F. G., M. Barker, L. W. Baillie, E. C. Holmes, and M. C. Maiden. 2004. Population structure and evolution of the Bacillus cereus group. J. Bacteriol. 186:7959-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radnedge, L., P. G. Agron, K. K. Hill, P. J. Jackson, L. O. Ticknor, P. Keim, and G. L. Andersen. 2003. Genome differences that distinguish Bacillus anthracis from Bacillus cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 69:2755-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramisse, V., G. Patra, J. Vaissaire, and M. Mock. 1999. The Ba813 chromosomal DNA sequence effectively traces the whole Bacillus anthracis community. J. Appl. Microbiol. 87:224-228. [DOI] [PubMed] [Google Scholar]
- 48.Rasko, D. A., M. R. Altherr, C. S. Han, and J. Ravel. 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 29:303-329. [DOI] [PubMed] [Google Scholar]
- 49.Rasko, D. A., J. Ravel, O. A. Økstad, E. Helgason, R. Z. Cer, L. Jiang, K. A. Shores, D. E. Fouts, N. J. Tourasse, S. V. Angiuoli, J. Kolonay, W. C. Nelson, A. B. Kolstø, C. M. Fraser, and T. D. Read. 2004. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic Acids Res. 32:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Read, T. D., S. N. Peterson, N. Tourasse, L. W. Baillie, I. T. Paulsen, K. E. Nelson, H. Tettelin, D. E. Fouts, J. A. Eisen, S. R. Gill, E. K. Holtzapple, O. A. Økstad, E. Helgason, J. Rilstone, M. Wu, J. F. Kolonay, M. J. Beanan, R. J. Dodson, L. M. Brinkac, M. Gwinn, R. T. Deboy, R. Madpu, S. C. Daugherty, A. S. Durkin, D. H. Haft, W. C. Nelson, J. D. Peterson, M. Pop, H. M. Khouri, D. Radune, J. L. Benton, Y. Mahamoud, L. Jiang, I. R. Hance, J. F. Weidman, K. J. Berry, R. D. Plaut, A. M. Wolf, K. L. Watkins, W. C. Nierman, A. Hazen, R. Cline, C. Redmond, J. E. Thwaite, O. White, S. L. Salzberg, B. Thomason, A. M. Friedlander, T. M. Koehler, P. C. Hanna, A. B. Kolstø, and C. M. Fraser. 2003. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423:81-86. [DOI] [PubMed] [Google Scholar]
- 51.Saile, E., and T. M. Koehler. 2002. Control of anthrax toxin gene expression by the transition state regulator abrB. J. Bacteriol. 184:370-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Turnbull, P. C. 1999. Definitive identification of Bacillus anthracis—a review. J. Appl. Microbiol. 87:237-240. [DOI] [PubMed] [Google Scholar]
- 54.Turnbull, P. C., R. A. Hutson, M. J. Ward, M. N. Jones, C. P. Quinn, N. J. Finnie, C. J. Duggleby, J. M. Kramer, and J. Melling. 1992. Bacillus anthracis but not always anthrax. J. Appl. Bacteriol. 72:21-28. [DOI] [PubMed] [Google Scholar]
- 55.Turnbull, P. C., N. M. Sirianni, C. I. LeBron, M. N. Samaan, F. N. Sutton, A. E. Reyes, and L. F. J. Peruski. 2004. MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J. Clin. Microbiol. 42:3626-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchida, I., J. M. Hornung, C. B. Thorne, K. R. Klimpel, and S. H. Leppla. 1993. Cloning and characterization of a gene whose product is a trans-activator of anthrax toxin synthesis. J. Bacteriol. 175:5329-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. 2003. Manual for laboratory diagnosis of anthrax. World Health Organization, Geneva, Switzerland. [Online.] http://w3.whosea.org/bct/anthrax/.