Abstract
The Helicobacter pylori cag pathogenicity island (cag PAI) encodes components of a type IV secretion system (T4SS) involved in host interaction and pathogenicity. Previously, seven cag PAI proteins were identified as homologs of Agrobacterium tumefaciens Vir proteins, which form a paradigm T4SS. The T pilus composed of the processed VirB2 pilin is an external structural part of the A. tumefaciens T4SS. In H. pylori, cag-dependent assembly of pili has not been observed so far, nor has a pilin (VirB2) ortholog been characterized. We have here identified, using a motif-based search, an H. pylori cag island protein (HP0546) that possesses sequence and predicted structural similarities to VirB2-like pilins of other T4SSs. The HP0546 protein displays interstrain variability in its terminal domains. HP0546 was expressed as a FLAG-tagged fusion protein in Escherichia coli, A. tumefaciens, and H. pylori and was detected as either two or three bands of different molecular masses in the insoluble fraction, indicating protein processing. As reported previously, isogenic H. pylori mutants in the putative cag pilin gene had reduced abilities to induce cag PAI-dependent interleukin-8 secretion in gastric epithelial cells. Fractionation analysis of H. pylori, using a specific antiserum raised against an N-terminal HP0546 peptide, showed that the protein is partially surface exposed and that its surface localization depended upon an intact cag system. By immunoelectron microscopy, HP0546 was localized in surface appendages, with surface exposure of an N-terminal epitope. Pronounced strain-to-strain variability of this predicted surface-exposed part of HP0546 indicates a strong selective pressure for variation in vivo.
The cag pathogenicity island (cag PAI) of Helicobacter pylori encodes proteins with homologies to structural and functional components of type IV secretion systems (T4SS) of other bacteria (1, 6, 15). These systems are multicomponent membrane-spanning transport systems dedicated to the secretion or translocation of high-molecular-mass biomolecules, such as protein-coupled DNA or proteins, into the environment or into recipient cells (8, 25, 27). The cag island is involved in the pathogenesis of gastric inflammation and gastric cancer in the human host (42). It enables the bacterium to translocate the CagA effector protein into host cells, which, as a consequence, causes a growth factor-like phenotype in infected epithelial cells (30, 31, 37). CagA is crucial for the development of cell morphology changes and the disruption of cell-cell contacts (tight junctions), effects which likely play a role in the development of ulcers and cancer during chronic H. pylori infection of the stomach. It is so far the only H. pylori macromolecule effector known to be translocated by the cag T4SS (3, 10, 17, 31, 43). In addition, the apparatus encoded by the cag PAI is instrumental in the induction of proinflammatory cytokines, such as interleukin-8 (IL-8), in human epithelial cells (39), which is a marker for host interaction and a hallmark of disease. This effect may be caused by muramyl tripeptide translocation and thereafter signal induction via the host pattern recognition protein NOD1 (45) and may be enhanced by the translocated CagA protein (4, 15, 38). Several H. pylori proteins encoded on the cag PAI were identified as homologs of Vir proteins from Agrobacterium tumefaciens, which constitute a model of T4SS (5, 6, 33). In this paradigmatic T4SS of A. tumefaciens, in addition to a complex transmembrane transport system, the membrane-associated and surface-exposed T pilus is an essential structural and functional component of type IV secreton (9, 27). The major subunit of the multimeric T pilus is the processed and cyclized pilin protein VirB2, which was found to participate in the translocation of the T-DNA-protein complex (25, 28). Up to now, neither a pilin ortholog in the complete genome sequences (2, 44) nor cag island-dependent or -independent assembly of typical pili has been detected in H. pylori. Recently, sheathed surface structures dependent on a functional cag pathogenicity island, not closely resembling classical T pili, but with a central pilus-like structure, have been detected in H. pylori (33, 43). In these studies, surface-exposed components of the pilus-like structures were identified as domains of cag-encoded proteins HP0527, HP0528, and HP0532 using immunogold labeling and electron microscopy (EM) (33, 43).
In our present study, we identified an H. pylori cag island protein (encoded by the HP0546 gene) that has significant similarity to VirB2-like pilins from other T4SS. The HP0546 protein was characterized to be important for the complete function of the cag T4SS in host interaction and to provide a structural component on the surfaces of the bacteria. The protein was expressed and detected in Escherichia coli, A. tumefaciens, and H. pylori. A strain-specific antiserum against an N-terminal peptide of HP0546 was generated and revealed that HP0546 was surface exposed in wild-type bacteria but not in a cag virB4 (HP0544) mutant. In electron microscopy, HP0546 (N-terminal epitope) was detected to be exposed at different sites on bacterial surfaces, within larger amorphous appendages. Taken together, the results suggest that the protein is a surface-associated VirB2-like pilin subunit that is functionally linked to the H. pylori cag apparatus.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. pylori N6 and KE 26695 wild-type strains, 88-3887 (the motile derivative of 26695), and a flagellumless fliP-negative derivative of N6 (14, 20, 44), which all harbor a functional cag secretion system, were used for protein analysis and for eukaryotic cell infections. NCTC11637, SS1, J99 (2), LSU2003, LSU1062-3, LSU1014, NQ315, RE10001, RE10002, and BO265 (13, 41), additional H. pylori strains of different geographical origins, each possessing a functionally intact cag island, were used for the preparation of proteins and extrabacterial appendages and for amplification and sequencing of the strain-specific HP0546 genes. H. pylori strains were cultured on blood agar plates (Columbia agar base II; Oxoid, Wesel, Germany) containing 10% horse blood and the following antibiotics: vancomycin (10 mg/liter), polymyxin B (2,500 U/liter), trimethoprim (5 mg/liter), and amphotericin B (4 mg/liter). H. pylori strains were preincubated on plates for 24 to 48 h at 37°C under microaerobic conditions for the infection assays. Mutant strains were propagated on blood agar plates with the addition of chloramphenicol (10 mg/liter) and/or kanamycin (100 mg/liter).
E. coli strains MC1061 and DH5α were used for the DNA cloning experiments and were propagated in Luria broth or on Luria-Bertani plates supplemented with kanamycin (100 mg/liter), chloramphenicol (20 mg/liter), ampicillin (100 mg/liter), and tetracycline (10 mg/liter) antibiotics as required.
The Agrobacterium tumefaciens bald strain NT1REB (without flagella) (24) was used for heterologous expression of HP0546.
Cell lines, growth conditions, and maintenance.
For infection with H. pylori, the human gastric carcinoma cell lines AGS and HM02 (isolated from adenocarcinoma of the stomach from Caucasian patients) and MKN28 (originally isolated from a Japanese cancer patient) (47) were used. Cells were routinely cultured in RPMI 1640 medium (buffered with 25 mM HEPES; Invitrogen) and supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) in a 6% CO2 incubator. Cells were subcultured every 3 to 4 days for maintenance.
Cell infections and determination of cytokine release.
Cell infections and coincubations with proteins were performed on subconfluent cell layers (epithelial cell lines, 70% to 90% confluent). Cells in 24-well tissue culture plates were washed three times and preincubated prior to infection for 30 min in fresh medium with serum and without antibiotics. At the time point of infection, mid-log-phase bacteria, washed and resuspended in fresh cell culture medium (RPMI 1640, 25 mM HEPES, 10% heat-inactivated FBS), were added at a multiplicity of infection of 50 bacteria per cell, and the incubation plates were centrifuged at 500 × g for 3 min to synchronize the infection. The coincubation was carried out for 20 h. Mock-infected cells were prepared as negative controls. For cytokine measurements, supernatants were harvested, cleared by centrifugation, and stored at −80°C until use. IL-8 release into the cell supernatants was quantitated using an OptEIA IL-8 enzyme-linked immunosorbent assay kit by BD Pharmingen (San Diego, CA) according to the manufacturer's instructions. Cell supernatants were used at appropriate dilutions for measurement. Each assay was performed in triplicate. Means and standard deviations for at least three independently performed coincubations were calculated. The level of confidence was set at 95%.
DNA methods.
DNA purification and cloning procedures were performed as described elsewhere (35). Large-scale plasmid purifications were performed using QIAGEN column purification protocols (QIAGEN, Hilden, Germany). DNA fragments were extracted from agarose gels using a QiaEX DNA purification kit (QIAGEN). DNA restriction and modification enzymes were obtained from Invitrogen Life Technologies or Roche Biochemicals and were used according to the manufacturer's protocols. Plasmids and oligonucleotide primers are listed in Table 1 and Table 2, respectively.
TABLE 1.
Plasmids used in this study
| Plasmid/vector | Size (bp) | Commentsa | Source or reference |
|---|---|---|---|
| pHel2 | 5,000 | Cmr, RepEc, RepHp, multicopy shuttle vector for E. coli and H. pylori | 18 |
| pILL600 | 5,700 | Ampr, Kmr, RepEc, source of Km resistance cassette | 23 |
| pUC18 | 2,690 | Ampr, RepEc, high-copy-number cloning vector | |
| pTrc99A | 4,584 | Ampr, RepEc, source of inducible Ptrc | |
| pCAMBIA-0,380 | 6,812 | Kmr, RepEc, RepAt, binary vector for A. tumefaciens | 16 |
| pCJ201-1 | 7,593 | Cmr, RepEc, RepHp, inducible Ptrc/promoterless HP0546 gene in same transcriptional orientation as Ptrc in pHel2 | This work |
| pCJ201-0 | 7,025 | Cmr, RepEc, RepHp, Ptrc in pHel2, control plasmid for pCJ201-1r and pCJ206 | This work |
| pCJ202-1 | 5,074 | Ampr, Kmr, RepEc, HP0546 disrupted by Km resistance cassette in pUC18 | This work |
| pCJ203-0At | Kmr, RepEc, RepAt, fusion of partial sequence of pCAMBIA-0380 with inducible promoter of vector pTrc99a to serve as inducible expression plasmid for A. tumefaciens | This work | |
| pCJ203-1r | 7,573 | Kmr, RepEc, RepAt, inducible Ptrc/promoterless HP0546 gene are in the same transcriptional orientation in pCJ203-0At | This work |
| pCJ203-1w | 7,573 | Kmr, RepEc, RepAt, inducible Ptrc/promoterless HP0546 gene are in transcriptional orientation opposite to that of promoter in pCJ203-0At (negative control for expression experiments) | This work |
| pCJ204 | 6,048 | Cmr, RepEc, RepHp, HP0546 in pHel2, control plasmid for pCJ205 (without FLAG tag) | This work |
| pCJ205 | 6,072 | Cmr, RepEc, RepHp, HP0546 with FLAG tag integrated into HP0546, cloned in pHel2, source of immunodetectable HP0546 fusion protein | This work |
| pCJ206 | 7,617 | Cmr, RepEc, RepHp, derivative of pCJ201-1 containing FLAG-tagged HP0546 fusion protein | This work |
| pCJ207 | 7,597 | Kmr, RepEc, RepAt, derivative of pCJ203-1r containing FLAG-tagged HP0546 fusion protein | This work |
Ampr, ampicillin resistant; Cmr, chloramphenicol resistant; Kmr, kanamycin resistant; RepEc, plasmid which can replicate in E. coli; RepHp, plasmid which can replicate in H. pylori.
TABLE 2.
Oligonucleotides used in this study for PCR and nucleotide sequence determination
| Primer | Purpose | Sequence (underlined site)a |
|---|---|---|
| HP546p1 (forward) | PCR | 5′-ATA GGA TCC TCT CAC TCT GAT CAG CTT GG-3′ (BamHI) |
| HP546p2 (reverse) | PCR | 5′-ATA GGA TCC TTC CTT TCA AAT TGA AAT CAA TCG-3′ (BamHI) |
| HP546_3 (forward) | PCR | 5′-ATA GGA TCC GAT GAA TGC AAG GTG AGT GC-3′ (BamHI) |
| HP546_4 (reverse) | PCR | 5′-AAT GGA TCC GAT AGT CGC CTT GAA ACT ATC-3′ (BamHI) |
| HP546_5 (forward) | PCR | 5′-AAT AGA TCT TGA TTT CAA TTT GAA AGG AAA CG-3′ (BglII) |
| HP546Nco_1 (forward) | PCR | 5′-TAT ACC ATG GTG ATT TCA ATT TGA AAG GAA ACG-3′ (NcoI) |
| HP546Nco_2 (reverse) | PCR | 5′-TAT ACC ATG GGA TAG TCG CCT TGA AAC TAT C-3′ (NcoI) |
| HP546inv_1 (forward) | PCR | 5′-ATA TCG ATC ATT TCT GTT CTA GCG ATC G-3′ (ClaI) |
| HP546inv_2 (reverse) | PCR | 5′-ATA TCG ATG ATT GGT TGT TAC CAC TAG C-3′ (ClaI) |
| HP546 FLAG1 | PCR | 5′-ATG GAT TAC AAG GAT GAC GAC GAT ATT CAG ATC ATT TCT GTT CTA GC-3′ (FLAG tag) |
| HP546 FLAG2 | PCR | 5′-ATC GTC GTC ATC CTT GTA ATC CAT AAC CAA AGT TTT AGT CTC AGT AAC-3′ (FLAG tag) |
| pCAM380_1 (forward) | PCR | 5′-ATA AGA TCT AGG ACG CAT TGA CCG AGG-3′ (BglII) |
| pCAM380_3 (reverse) | PCR | 5′-ATA AGA TCT GAA CAG TGA ATT GGA GTT CG-3′ (BglII) |
| pTrc991 (forward) | PCR | 5′-ATA AGA TCT TCA CCG TCA TCA CCG AAA CG-3′ (BglII) |
| pTrc992 (reverse) | PCR | 5′-ATA AGA TCT GAG TTT GTA GAA ACG CAA AAA GG-3′ (BglII) |
| lac_pHel | Nucleotide sequencing | 5′-ACT TCC AGT TCA ACA TCA GC-3′ |
| km1 | Nucleotide sequencing | 5′-CTG CTA AGG TAT ATA AGC TGG TGG G-3′ |
| km2 | Nucleotide sequencing | 5′-CAT ACT GTT CTT CCC CGA TAT CCT C-3′ |
| pTrc_MCS2 | Nucleotide sequencing | 5′-ACT TCT GAG TTC GGC ATG G-3′ |
| pUC/M13 (reverse) | Nucleotide sequencing | 5′-TCA CAC AGG AAA CAG CTA TGA C-3′ |
The site in parentheses is represented by the underlined nucleotides.
Protein methods and Western blotting.
Protein concentrations were determined by Bradford (Bio-Rad protein assay dye reagent; Hercules, CA) or bicinchoninic acid (Pierce, Rockford, IL) assay. Equal amounts of protein per sample were separated on 16.5% denaturing Tris-Tricine sodium dodecyl sulfate (SDS)-polyacrylamide gels or on 14% Tris-glycine SDS-polyacrylamide gel electrophoresis (PAGE) gels and, for Western blotting, transferred to nitrocellulose membranes of 0.2-μm pore size (Schleicher & Schuell, Germany). The membranes were blocked in Tris-buffered saline-0.1% Tween 20 or in Tris-buffered saline-0.1% Tween 20 with 5% nonfat dried milk for 1 h and afterwards incubated sequentially with anti-FLAG tag mouse monoclonal antibody M2 (dilution, 1:500; SIGMA) or affinity-purified anti-HP0546 polyclonal antiserum (dilution, 1:1,000) overnight at 4°C and then peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) or alkaline phosphatase-conjugated goat anti-mouse IgG (dilution, 1:50,000 or 1:5,000; Jackson ImmunoResearch Laboratories) for 2 h. Immunolabeled proteins were revealed using Nitro Blue Tetrazolium-BCIP (5-bromo-4-chloro-3-indolyphosphate) color detection or chemiluminescence substrate Enhanced SuperSignal West (Pierce).
Isolation of H. pylori surface appendages.
H. pylori surface appendages, containing flagella and other surface-associated material, were isolated as previously described (20). Briefly, cells were grown to late log phase, harvested from blood agar plates, and subjected to mild shearing forces by repeated (20 times) pushing of the suspension through 26-gauge syringe needles. Intact bacterial cells and the sheared-off material were separated by differential centrifugation steps (first at 8,000 × g for 20 min and then at 40,000 × g for 1 h in an Optima CU-TL100 Beckman ultracentrifuge), and the protein content of the preparations was determined. The preparations were also evaluated by electron microscopy and contained predominantly flagella in flagellated strains (confirmed by Western immunoblotting using flagellin-specific antisera), which indicated that the protocol successfully isolates surface-associated material.
Production of a HP0546-specific antiserum.
A peptide of HP0546 (strain 26695) was chosen, according to published material on various VirB2-like proteins (27), for the production of an antiserum. We selected the peptide TSPAEGVTETKTLVIQ (16 amino acids [aa]) at the N terminus of the predicted mature HP0546 protein, immediately following the putative leader peptide cleavage site (amino acids 31 to 46 of the predicted HP0546 protein), since it has been suggested that in other bacteria (E. coli [11, 29, 34] and A. tumefaciens [25]), the N termini of the processed VirB2-like pilin proteins may be surface exposed in the correctly folded mature pilin subunits and in the pilus polymers. The peptide was synthesized (Biosyntan/Biogenes, Berlin, Germany), and two rabbits were subcutaneously injected with the peptide conjugated to a carrier to increase its immunogenicity. After four booster injections, the serum was obtained and affinity purified using the specific peptide. Western blots of whole cells and membrane preparations of H. pylori were used as controls for the specificity of the peptide antibody and recognized only a single protein species (see Results).
Deletion and insertion mutageneses of HP0546 and HP0544 genes.
For the further functional characterization of HP0546, mutations in the HP0546 gene were constructed by gene deletion and replacement and subsequent allelic exchange in different H. pylori strains. The HP0546 gene with its own promoter region (1,048 bp) was amplified from strain 26695 using primers HP0546_10 and HP0546_4 (BamH1 sites) and cloned into pUC18 (plasmid pCJ202-0). Inverse PCR of the whole pCJ202-0 plasmid was performed using primers HP0546inv_1 and HP0546_2 (ClaI sites). Thereby, an almost complete deletion of the HP0546 coding sequence was engineered, in order to exclude the generation of a partial protein with residual function, while preserving possible regulatory sequences for downstream genes at the 3′ end of the coding sequence. A kanamycin resistance cassette (aphA3′-III, derived from pILL600) (23) was inserted into the inversely amplified plasmid in the same transcriptional orientation as the gene, resulting in pCJ202-1. Subsequently, plasmid pCJ202-1 was introduced into H. pylori strains N6, 26695, and 88-3887 (the motile derivative of 26695) by natural transformation. The HP0544 (cag island virB4) gene was inactivated in strain 88-3887 by a rapid method without cloning steps, using three subsequent PCRs and transforming final PCR products into H. pylori wild-type strains for marker exchange mutagenesis (details of mutant construction available on request). Correct replacement of the target genes in the chromosome by double crossover and marker exchange by directional integration of the kanamycin resistance cassette were determined by PCR using different primer combinations.
Expression of the H. pylori HP0546 protein in H. pylori, A. tumefaciens, and E. coli and engineering of HP0546 internal FLAG tag fusion constructs.
For expression of HP0546 in H. pylori, the gene containing its own promoter (PCR product generated using 26695 genomic DNA as a template and primers HP0546_3 and HP0546_4 [BamHI sites]) was cloned as a 586-bp fragment into the H. pylori/E. coli shuttle plasmid pHel2 (18). The resulting plasmid was pCJ204. An inducible plasmid used for expression of the HP0546 protein in A. tumefaciens was constructed as follows. A 2,025-bp fragment of pTrc99A with the inducible trc promoter was amplified (primers pTrc99A_1s and pTrc99A_2s), gel purified, and cut using BglII. It was ligated with a 4,980-bp BglII fragment from the binary plasmid pCAMBIA-0380 for E. coli and A. tumefaciens (16), which was generated by PCR amplification (primers pCAM380_1 and pCAM380_3 [Table 2]). The resulting plasmid, pCJ203-0, contains replication origins for A. tumefaciens and for E. coli, a kanamycin resistance gene (aadA) for selection, the lacIq gene encoding the Lac repressor, the inducible trc promoter of pTrc99A with a multiple cloning site, and strong transcriptional terminators (rrnB) downstream of the multiple cloning site. HP0546 was cloned into pCJ203-0 (cut with Nco1) as a 567-bp fragment, exclusive of its own promoter, generated by primers HP0546Nco_1 and HP0546Nco_2 (NcoI sites). Genomic DNA of H. pylori 26695 was used as a template. The resulting plasmids were pCJ203-1r and pCJ203-1w (inverse orientation of insert, used as a negative control). The insertion site and the orientation of the HP0546 gene relative to the trc promoter in pCJ203-1r and pCJ203-1w were confirmed by sequencing. Plasmid pCJ205, containing the HP0546 gene downstream of an inducible Ptrc promoter in pHel2, was constructed by insertion of the Nco1-amplified HP0546 fragment as described above into the pCJ201 plasmid (unpublished), to yield pCJ201-1. In addition, plasmids pCJ204, pCJ203-1, and pCJ201-1 were all engineered by PCR mutagenesis to contain an in-frame fusion with a FLAG tag sequence (MDYKDDDD) within the coding region of the HP0546 amino acid sequence (between aa 44 and aa 45) (Fig. 1B), to permit detection of the HP0546 gene in Western blots (resulting plasmids were pCJ205, pCJ207, and pCJ206 [Table 1] [details of construction available on request]). The correct FLAG tag fusion was confirmed by sequence analysis of all plasmids. For expression analyses, all plasmids were transformed into the appropriate host bacteria (H. pylori N6, H. pylori 26695, E. coli MC1061, and A. tumefaciens NT1REB). All pTrc-derivative plasmids for inducible expression in E. coli and A. tumefaciens were constructed such that they should allow the expression of the HP0546 protein from its own start codon without any additional N- and C-terminal amino acids.
FIG. 1.
Alignment of H. pylori HP0546 protein (strain 26695) with various known type IV pilins of other bacteria. (A) The alignment shown was created with CLUSTAL W and revised manually (see Materials and Methods). Designations of protein origins, protein names, and bacterial species/plasmids are shown to the left of each alignment. Abbreviations for the plasmids/bacterial species: pKm10, pKM10 of E. coli; RP4, RP4 of E. coli; R388, R388 of E. coli; Eco, E. coli; atu, A. tumefaciens; bsui, Brucella suis; bhe, Bartonella henselae; borpe, Bordetella pertussis; Lepn, Legionella pneumoniae; Hpyl, H. pylori. Shading of the amino acids according to their physicochemical properties was performed with the GeneDoc software (www.psc.edu/biomed/genedoc). (B) The manual alignment of H. pylori HP0546 with the A. tumefaciens VirB2 type IV pilin protein shows 20% amino acid identity. The leader peptide cleavage site (putative for HP0546, at A29), the insertion site of an internal FLAG tag epitope (between V44 and I45) in HP0546, and the peptide (T31 to Q47; bold and underlined) which was used for generation of the specific antiserum are indicated in panel B.
Complementation of the HP0546 gene in H. pylori.
For complementation, HP0546 plasmid pCJ204 (pHel2 derivative), including its own 5′ untranslated region, containing putative promoter sequences, was transformed into the H. pylori N6 wild-type strain and the H. pylori 88-3887 isogenic HP0546 mutant. In cases where transformants were obtained on chloramphenicol plates, production of the HP546 protein was determined by Western blotting.
Fluorescent labeling of cells and microscopy.
Immunofluorescent labeling of surface-exposed HP0546 on H. pylori in the presence and in the absence of human epithelial cells grown on gelatin-coated coverslips was performed using affinity-purified HP0546 antiserum (dilution, 1:1,000). Binding of primary antibody was detected using Alexa Fluor 488-coupled goat anti-rabbit secondary antibody (dilution, 1:5,000; Molecular Probes). The samples were viewed in a Leica TCS confocal microscope and the TIFF image files processed with Adobe Photoshop.
Electron microscopy.
H. pylori and infected cells were prepared for electron microscopy as follows. Cells were seeded in drops of cell medium on EM gold grids (300 mesh, Formvar-carbon coated; Plano, Germany), which had been cleansed and sterilized by being rinsed for 1 h in 70% ethanol of the purest grade in bidistilled H2O. Cells were grown overnight in RPMI 1640-25 mM HEPES-10% FBS in a humid chamber (using a process similar to that described in reference 19) and then washed once and overlaid carefully with drops of fresh medium supplemented with 10% FBS. Cells were infected with a droplet of H. pylori bacteria resuspended in cell culture medium to an optical density at 600 nm of approximately 1.0. Cells were coincubated with the bacteria for 2 or 3 h. Then, the medium was removed gently, and fixing agent (2% paraformaldehyde in 100 mM phosphate buffer, pH 7.0) was layered on the grids for 20 min. Subsequently, the grids were treated for immunolabeling with the affinity-purified anti-HP0546 antiserum (1:100) in 2% goat serum-2% fish skin gelatin-2% bovine serum albumin in phosphate-buffered saline, pH 7.4, for 30 min. After appropriate washing steps in 0.1% bovine serum albumin in phosphate-buffered saline, the primary antibody was detected by incubation in goat anti-rabbit IgG secondary antibody coupled to 10 nm colloidal gold (1:80). After final washing steps and a secondary fixation in 2.5% glutaraldehyde for 10 min, the grids were negatively stained using 1% phosphotungstate, pH 7.0. The grids were viewed using a Zeiss EM-10 transmission electron microscope, and pictures were taken on Kodak EM film and digitized by high-resolution scanning.
Bioinformatic analyses.
Identification of a putative pilin of the H. pylori cag PAI T4SS was performed by in silico analyses; pilins of nine different T4SS were aligned using CLUSTAL W software (GCG Wisconsin Package) and subsequently used as an input file for the program ProfileMake (GCG), which defines specific amino acid motifs of a homologous protein family. Pilins from the following systems were compared: (i) plasmid pKM101, E. coli (TraM); (ii) plasmid RP4, E. coli multiresistance plasmid (TrbC); (iii) E. coli plasmid R388 (TrwM); (iv) plasmid IncF1 R386, E. coli (TraA); (v) Agrobacterium tumefaciens (VirB2); (vi) Brucella suis (VirB2); (vii) Bartonella henselae (VirB2); (viii) Bordetella pertussis (PtlA); and (ix) Legionella pneumophila (LvhB2). The output file of the pilins created by the ProfileMake software was further analyzed by performing ProfileSearch (GCG) on the PIR and Swiss-Prot databases. In the subsequently received output file, the derived HP0546 protein of the H. pylori cag PAI was identified as one single H. pylori protein containing amino acid motifs of a possible T4SS pilin. Subsequently, the HP0546 protein was automatically (MULTALIGN, CLUSTAL W) and further manually aligned with the nine other pilins using the GeneDoc software (www.psc.edu/biomed/genedoc) (Fig. 1A). The amino acid hydrophobicity profile in comparison to those for other VirB2-like pilins was tested in silico using a membrane protein hydrophobicity algorithm (12).
RESULTS
Identification of a virB2-like gene (HP0546) in the H. pylori cag pathogenicity island.
The Vir system of A. tumefaciens, which transports Ti plasmid transfer DNA into plant cells, in association with specific Vir proteins, is a prototype of the type IV apparatus (27), of which structural proteins VirB1 through VirB11 and VirD4 are the essential constituents (22). The T pilus, a multisubunit structure predominantly consisting of VirB2 subunits, forms an important part of the VirB T4SS (9). In H. pylori, neither a T4SS-associated pilus nor a VirB2-like pilin subunit associated with the cag T4SS has been characterized so far. We identified the cag PAI-encoded HP0546 (Cag25, CagC) as a candidate VirB2-orthologous pilin protein of the cag apparatus in H. pylori using a database search, with peptide motifs compiled from amino acid sequences of nine known orthologous pilins of T4SS from different bacteria (Fig. 1A), as detailed in Materials and Methods. The 115-aa predicted full-length HP0546 protein with its putative leader peptide (see below) has an overall amino acid identity of 19% (CLUSTAL W) to the A. tumefaciens VirB2 protein (Fig. 1). T4SS pilins of other bacteria show low overall amino acid identities to each other and to the prototype VirB2 protein of A. tumefaciens, but all display a characteristic hydrophobicity profile, with two hydrophobic peaks in the mature polypeptide, after cleavage of a leader peptide, indicating hydrophobic or putative transmembrane alpha helices. Hydrophobicity plotting (not shown) revealed that the HP0546-derived protein (when the putative leader peptide cleaved between aa 29 and 30 is omitted) contained two hydrophobic domains (aa 35 to 47 and aa 72 to 95), very similar to other structurally well-characterized VirB2-like pilins (Fig. 1). By means of alignment with other pilins (Fig. 1), the potential leader peptide cleavage site of HP0546 was modeled. The A. tumefaciens VirB2 pilin and related proteins are expressed as propilins and are processed at their amino and carboxy termini before integration into the pilus polymers (9, 25). In A. tumefaciens VirB2, a leader peptide is cleaved off between a stretch of three alanine residues at positions 45 to 47 and the adjacent glutamine residue at position 48. By analogy, we positioned the putative cleavage site in HP0546 between Ala (position 29) and Val (position 30), which represents a hydrophilic amino acid stretch between two hydrophobic domains. In Fig. 1B, the leader peptide cleavage sites in HP0546 (putative) and in A. tumefaciens VirB2 are indicated. The proposed leader peptide in HP0546 is shorter than that in A. tumefaciens VirB2 but comparable to those in other pilins of the VirB2 family (Fig. 1A). The putative mature HP0546 protein after cleavage was calculated to have a molecular mass of 9.7 kDa (as opposed to 13 kDa with the leader peptide).
To ascertain that a complete HP0546-like sequence is contained in cag PAIs of different H. pylori isolates and to gain an overview of the possible diversity of this gene and its derived protein, the nucleotide sequences of HP0546 from various strains from different geographical origins were amplified by PCR and determined (see Materials and Methods; also data not shown). The gene was present and complete in all (12) strains investigated and showed synonymous and nonsynonymous strain-to-strain variation. The derived amino acid sequences of the HP0546 proteins of these 12 H. pylori strains were aligned and compared (see Fig. S1 in the supplemental material). Amino acid variation was found within the putative leader peptide (aa 1 to 29), at the proposed mature N terminus following the putative leader sequence (aa 29 to 40), and also within the last four amino acids at the C terminus (aa 110 to 115). We then set out to characterize further the expression and localization of the pilin ortholog HP0546 by expressing and characterizing the protein in three bacterial species which can assemble T4SS of different functions, namely, in E. coli, A. tumefaciens, and H. pylori.
HP0546 was expressed and processed in E. coli, A. tumefaciens, and H. pylori and localized to the insoluble (membrane-associated) fraction.
The HP0546 protein was first expressed in E. coli MC1061 using the three plasmids pCJ206, pCJ207, and pCJ205, each containing an internally FLAG-tagged in-frame gene fusion of HP0546 (Materials and Methods and Fig. 1B). We thereby tested protein expression from all of these plasmids in E. coli before these plasmids were used further as inducible plasmids in E. coli(pCJ206) and A. tumefaciens(pCJ207) and as noninducible expression plasmids in H. pylori(pCJ205). After induction of the expression plasmids pCJ206 and pCJ207 by IPTG (isopropyl-β-d-thiogalactopyranoside) (see Materials and Methods), harvested cells were fractionated into surface-exposed proteins (shearing) as well as insoluble and soluble fractions by ultrasonication. The three fractions were analyzed by Tris-Tricine SDS-PAGE and subsequent Western blotting against the FLAG tag (Fig. 2A).
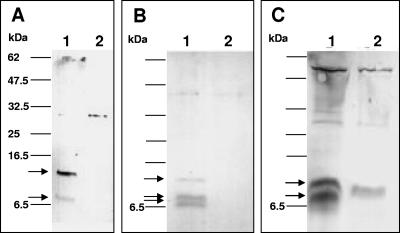
FIG. 2.
Expression of FLAG-tagged HP0546 in three different bacterial hosts, E. coli, A. tumefaciens, and H. pylori, reveals that the protein is localized in the insoluble bacterial fractions and is processed. (A) Expression of HP0546 (of strain 26695) in E. coli MC1061. (B) Expression of HP0546 in A. tumefaciens NTREB1. (C) Expression of HP0546 in H. pylori 26695. Proteins (5 μg per lane) were separated on 16.5% Tris-Tricine SDS-PAGE gels, and Western blots were developed with anti-FLAG tag M2 monoclonal antibody. Only proteins from insoluble fractions after ultrasonication are shown, since no signals were detected in the soluble fractions. Lanes 1, insoluble fractions, which show HP0546 protein expression (plasmid constructs used for HP0546 expression are pCJ206 [A], pCJ207 [B], and pCJ205 [C]); lanes 2, insoluble fractions of negative controls without expression of specific FLAG-tagged proteins (plasmids used as negative controls are pCJ201-0 [A], pCJ203-1w [B], and pCJ201-0 [C]). Molecular masses of standard proteins are indicated on the left; arrows indicate proteins specifically detected with the anti-FLAG tag antibody.
Expression levels and band patterns of the HP0546 protein in E. coli were found to be similar in Western immunoblots (anti-FLAG) after induction of plasmids pCJ206 (Fig. 2A) and pCJ207 (not shown). In the immunoblots, two bands that were not present in the negative controls were detected in the insoluble fractions after ultrasonication (pCJ201-0 not containing the HP0546 insert) (Table 1 and Fig. 2A). The two bands, including the FLAG tag (1 kDa), had apparent molecular masses of between 7 kDa and 16.5 kDa, which corresponded to masses calculated from the amino acid sequence with and without the putative leader peptide (13 kDa and 9.7 kDa [see above]). The two bands might be indicative of a processing of the protein in E. coli (see Discussion). No HP0546 protein bands were detected in the soluble fraction of E. coli cells under these conditions or in the fractions after mechanical shearing (surface-associated material). When HP0546 was expressed under the control of its own promoter in E. coli (plasmid pCJ205), only a low expression level was detected in Western blots (not shown).
Our aim was also to express HP0546 in A. tumefaciens, since the Vir T4SS encoded on the Agrobacterium Ti plasmid is very well characterized, and the conditions of the expression of its components are well established (24), which may offer improved methods for further investigating the functions of the protein (27). We also wanted to know whether processing of HP0546 observed in E. coli MC1061, a K-12 E. coli strain less well characterized for T4SS functions, also occurs in A. tumefaciens. For heterologous expression of HP0546, we used the A. tumefaciens bald strain NT1REB, which does not contain the pTi and does not form flagella (7). In the presence of pTi, this strain is able to form T pili from fully processed VirB2 (11), making this strain suitable for characterization of the orthologous HP0546 protein in the absence of flagella. A. tumefaciens strain NT1REB was transformed with plasmids pCJ203-1r (control for HP0546 without a FLAG tag) and pCJ207, which encodes FLAG-tagged HP0546. A. tumefaciens NT1REB(pCJ207) and controls were induced in liquid culture (0.5 mM IPTG, 200 mM acetosyringone, 19°C, 2 to 3 days), separated into soluble, insoluble, and sheared fractions by sonication, and analyzed by immunodetection on Western blots using anti-FLAG tag antibody (Fig. 2B). HP0546 was detected exclusively in the insoluble fraction of A. tumefaciens NT1REB(pCJ207). In contrast to expression in E. coli, three HP0546-specific bands, of which the two smaller bands appeared to have very similar molecular masses, were labeled using the FLAG tag antibody. As in E. coli, HP0546 was not detected in the soluble and surface-associated fractions. These results confirmed the functionality of the expression plasmid pCJ203 for A. tumefaciens and showed that HP0546 is expressed mostly as an insoluble protein (putatively membrane associated) and is processed in A. tumefaciens. This bears the potential for further functional and complementation studies of the HP0546 protein in the heterologous system of VirB2-deficient A. tumefaciens strains.
One further goal was the overexpression and detection of HP0546 in H. pylori, in order to study its localization, processing, and function in the native system. pCJ205 (HP0546 with an internal FLAG tag under the control of its own promoter, which, due to an approximately fivefold gene copy effect, should permit a slight overexpression of the protein) and pCJ201-0 (negative control) were transformed into the H. pylori N6 and N6 fliP (flagellumless mutant) strains. Soluble and insoluble proteins and surface-associated materials were prepared as described above for E. coli and A. tumefaciens. Immunoblot analyses performed with anti-FLAG tag antibody revealed two specific bands, which were not present in the negative controls, in the insoluble fraction of H. pylori N6(pCJ205) (Fig. 2C). The molecular masses of the two protein bands appeared to be similar to those for E. coli MC1061(pCJ206) (Fig. 2). In the soluble fraction and in material sheared from the surfaces, again, no bands were detected with the anti-FLAG antibody (not shown).
To investigate the possibility of multimer formation by HP0546, nondenaturing PAGE was performed and further analyzed by Western immunoblotting. No signal was obtained after immunoblotting with anti-FLAG tag antibody (not shown), which suggests that the internal FLAG tag is not accessible in native HP0546 in monomeric or multimeric state.
Immunodetection after denaturing PAGE was also performed for samples of the H. pylori N6(pCJ205) fliP mutant after introduction of the HP0546 expression plasmid, a flagellar basal body mutant not able to form flagellar appendages, which we used in order to facilitate detection in electron microscopy of small surface appendages. One single specific band, which corresponded to the smaller band detected in the insoluble fraction of H. pylori N6(pCJ205) (Fig. 2C), was detected in the insoluble fraction by anti-FLAG tag antibody in this mutant (not shown).
In Coomassie blue-stained SDS-PAGE, no differences in band pattern were observed between HP0546-positive samples of E. coli, H. pylori, and A. tumefaciens and their respective negative controls, indicating an overall low expression level of HP0546 in these expression systems under in vitro growth conditions (not shown).
Identification of HP0546 in H. pylori by a specific antiserum.
A specific antiserum against HP0546 was produced, since the anti-FLAG tag antibody proved to be of limited sensitivity and specificity for the detection of HP0546 in its native from and in microscopy (data not shown). The HP0546-specific antiserum was raised in rabbits against a specific N-terminal peptide of HP0546 from H. pylori 26695 (Materials and Methods), which, as we hypothesized, may be surface associated in mature pilin protein (see Discussion). To aid in specific immunodetection by microscopy, the serum was affinity purified by column chromatography using the same HP0546 peptide. In Western blots of denaturing PAGE, the affinity-purified anti-HP0546 peptide antiserum recognized one single band (with a molecular mass of approximately 10 kDa, corresponding to the faster-migrating band in the FLAG-tagged HP0546 expression strains [see above]) in lysates of heterologous H. pylori strain N6 and in E. coli, both expressing the HP0546 of strain 26695 from a plasmid (Fig. 3A). Otherwise, in the absence of plasmid expression, the specific antiserum detected the HP0546 protein only in the wild-type H. pylori strain 26695 and its motile variant 88-3887 (Fig. 3A and B), not in strain N6 or in the more than 10 additional H. pylori strains tested (Fig. 3A and not shown). In some Western blots, in addition to the 10-kDa band, a high-molecular-mass band (>100 kDa) was weakly detected in the insoluble fraction of HP0546-overexpressing H. pylori, using the specific serum (Fig. 3A). The specific bands were also not detected in the HP0546 mutants of 26695 and 88-3887 (Fig. 3A and B; also see below), which confirmed the specificity of the detection and the successful mutagenesis of the gene. Further analysis of the HP0546 nucleotide and derived amino acid sequences in several strains and repeated Western blotting of various strains with different HP0546 sequences showed that the N-terminal peptide-specific antiserum was entirely strain specific for strains 26695 and 88-3887. In an additional collection of 30 different H. pylori strains from different geographical origins (B. Linz, unpublished), we could find only one single strain which had exactly the same N-terminal HP0546 sequence adjacent to the putative leader peptide as strain 26695. However, this strain (M49) did not possess a functional cag PAI and apparently did not express the protein. When we looked again in more detail for localization of native HP0546 with this high-affinity and high-specificity antiserum in fractionated bacteria of strain 88-3887, we could detect the protein most strongly in the insoluble fraction and in surface-associated material sheared from intact bacteria of the specific strain but also in smaller amounts in the soluble fraction (Fig. 3A and B). When bacteria were incubated for 1 h at 37°C in cell culture medium in the presence of lysed AGS cells, the protein was expressed in amounts similar to those for plate-grown bacteria, but under the former conditions, shearing of surface material yielded much less HP0546 protein than it did from plate-grown bacteria (not shown). Successful bacterial fractionation was confirmed with antisera against proteins specific for the soluble bacterial cytoplasmic fraction (UreB urease subunit), the membrane fraction (antiserum against flagellar basal body protein FlhA), and the surface-associated fraction (FlaB flagellin) of H. pylori (Fig. 3B). Another novel observation in the bacterial fractions analyzed using the strain-specific antiserum was that HP0546 protein in the surface-associated fraction migrated at a slightly higher molecular mass in SDS-PAGE than cytoplasmic or membrane-associated HP0546 (Fig. 3B). Flagellumless H. pylori 88-3887 fliP mutants also expressed the HP0546 protein, like the wild type (Fig. 3B). These results obtained with the specific antiserum extended our localization studies of overexpressed Flag-tagged HP0546 in a heterologous H. pylori strain and in other bacterial species.
FIG. 3.
Strain-specific detection of HP0546 protein in H. pylori using an antiserum raised against a peptide. Western blotting and immunolabeling of HP0546 (with and without FLAG tag), expressed in different H. pylori wild-type and plasmid-transformed strains and detected by a strain- and epitope-specific antiserum raised against an N-terminal peptide of HP0546, and control samples are shown. (A) Expression and strain-specific detection of HP0546 (from strain 26695) in E. coli MC1061 and in different H. pylori strains and mutants, including negative control samples which either do not express the protein (HP0546 mutant) or express a protein with a different N-terminal sequence (N6 and the N6 fliP mutant). Ten micrograms of protein (whole bacterial lysates) was loaded in each lane. (B) Western blot on which fractions of strain 88-3887 and its fliP (devoid of flagella), HP0546, and HP0544 mutants were analyzed, using the strain-specific anti-HP0546 antiserum. Insoluble (membrane) (I), soluble (cytoplasmic) (S), and surface-associated (extracellular) (E) bacterial fractions are depicted. HP0546 protein was found in the wild type in all three fractions, with predominance in the insoluble fraction. Note the slightly higher molecular mass of surface-associated HP0546. Four micrograms of protein was loaded in each lane. The upper two panels show fractionation controls for the same blot, developed using both anti-HPFlhA antiserum (raised against the membrane marker protein FlhA of the flagellar basal body) and anti-HPFlaB antiserum (raised against the extracellular marker protein flagellin B [20]). Arrows indicate protein bands, which were specifically detected by the respective antisera. Bands of lower molecular mass detected on FlhA-coincubated membranes and very weak bands detected by anti-HPFlaB are nonspecific bands.
Functional characterization of HP0546 by mutagenesis.
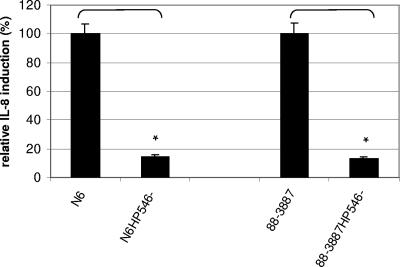
The HP0546 protein was inactivated in three different H. pylori strains (26695, 88-3887, and N6) by allelic exchange mutagenesis (see Materials and Methods). In the 26695 and 88-3887 HP0546 mutants, the lack of expression of the HP0546 protein was confirmed by Western blotting with the epitope-specific antiserum (Fig. 3A and B). Since the peptide antiserum produced against the HP0546 protein was specific for strains 26695 and 88-3887 (but did not react with strain N6 [see above]), it was not possible to confirm by immunoblot analysis the expected lack of the protein in the H. pylori N6 HP0546 mutant. No obvious morphological or cell adherence differences between the mutants and the respective wild-type strains were observed with or without coincubation with human gastric epithelial cells (electron microscopy and adherence assays [data not shown]). Mutants of N6 and 26695/88-3887 in HP0546 showed strongly diminished abilities to induce IL-8 secretion in gastric epithelial cells in comparison to the wild-type strains (IL-8 secretion levels were approximately 15% of the wild-type level for the HP0546 mutants in both strains) (Fig. 4), as was previously described for different H. pylori strains and their isogenic HP0546 mutants (15, 38). Functional complementation of the loss of HP0546 by transformation of the HP0546 mutant of strain 88-3887 with HP0546-containing shuttle plasmids (pCJ204 or pCJ205 [Table 1]) was not achieved, since no transformant clones were obtained after five attempts at natural transformation and electroporation in strain 88-3887, whereas transformants leading to heterologous protein expression were obtained for H. pylori strain N6 (Fig. 3A), which has a much higher transformation efficiency.
FIG. 4.
Role of HP0546 in cell interaction. IL-8 release is reduced in HP0546 mutants compared to that in the isogenic wild-type 88-3887 (26695) and N6 strains. Human gastric epithelial cell lines (AGS for the 88-3887 strain pair and HM02 for the N6 strain pair) were coincubated for 20 h with the bacterial strains, and IL-8 secretion in the supernatants was measured by enzyme-linked immunosorbent assay. At least three independent cell infections were performed for each isogenic wild-type/mutant strain pair, and means/standard deviations for triplicate measurements of these triplicate infections are depicted. The results are summarized as relative IL-8 induction values (percentages of those for the respective wild-type strains, which were each set to 100%). Background values for mock-infected cells were deduced from all other values before the calculation. *, statistically significant differences in IL-8 induction between wild-type strains and isogenic mutants (paired t test; P < 0.005).
H. pylori HP0546 was localized by microscopy on the surfaces of intact bacteria using the epitope-specific antiserum.
For the next step, we used the purified specific antiserum to reveal the localization of HP0546 in intact wild-type bacteria using specific immunolabeling and microscopy. Immunofluorescence microscopy of fixed, intact H. pylori bacteria labeled with the HP0546-specific, affinity-purified antiserum confirmed the assumed surface localization that had been suggested by the previous fractionation experiments and showed a patchy distribution of HP0546-specific signal on the surfaces of H. pylori 26695 and 88-3887 bacteria (Fig. 5A). HP0546-specific labeling on bacterial surfaces was observed regardless of whether the bacteria were cultured in the presence or absence of eukaryotic cells, but visual inspection suggested higher surface expression levels of the protein in bacteria adhering to cells (Fig. 5A). No signal on bacterial surfaces or elsewhere was observed with the HP0546-specific antibody in fluorescence microscopy samples of 88-3887 HP0546 mutants (Fig. 5B) or of human AGS cells alone. Immunogold labeling of specimens for electron microscopy confirmed this result and showed that amorphous appendages on the surfaces of H. pylori bacteria were labeled by the specific antiserum (Fig. 6). Bacteria clearly carried flagella and HP0546-containing material on their surfaces simultaneously (Fig. 6). Specimens of HP0546 mutants and control specimens of cells without bacteria, which were incubated with secondary antibody only, had bound less than one gold particle per 100 μm2, demonstrating a high specificity of detection with the specific antiserum. These results clearly demonstrate that HP0546 is a surface-associated protein of intact H. pylori and that the peptide-specific antiserum recognizes a surface-exposed domain of the protein in a mature structure at least partially composed of HP0546 protein. We next tested whether surface localization of HP0546 is dependent on the presence of a functional cag T4SS. To this end, we constructed a cag island virB4 (HP0544, cagE) ATPase mutant deficient in cag system function (as indicated by its reduced ability to induce IL-8 secretion in AGS cells). The mutant still expressed HP0546 in amounts similar to those for the wild-type strain, but HP0546 protein was not detected on bacterial surfaces by either fractionation (Fig. 3B) or immunofluorescence (not shown). In this mutant, higher amounts of cytoplasmic HP0546 were detected (Fig. 3B).
FIG. 5.
HP0546 is localized on bacterial surfaces using immunofluorescence microscopy of H. pylori specimens during cell infection, labeled with specific anti-HP0546 peptide antibody. (A) AGS cells infected with H. pylori 88-3887 wild-type bacteria for 4 h. (B) AGS cells coincubated with H. pylori 88-3887 HP0546 mutant bacteria for 4 h. Nonpermeabilized specimens were then stained by a lectin to visualize the AGS cell surface using wheat germ agglutinin coupled to Texas Red and subsequently fixed and immunolabeled using anti-HP0546 peptide antiserum (1:1,000), followed by anti-rabbit IgG coupled to Alexa Fluor 488 (1:5,000). Specimens were analyzed using confocal laser scanning microscopy, and representative focal planes are depicted as overlays between signals detected in the green (HP0546-specific signal) and red (cell surface staining) emission channels, combined with a differential interference contrast image of the same specimen. White bars are a size marker of 5 μm. No signal in the green channel (HP0546-specific) was detected in panel B.
FIG. 6.
Electron microscopy using anti-HP0546 antibody for immunolabeling of cag-positive H. pylori 88-3887 bacteria reveals surface-associated material. H. pylori bacteria were coincubated with AGS cells on electron microscopy grids for 2 h and then fixed and immunolabeled using gold-coupled secondary antibodies as described in Materials and Methods. Two representative bacterial specimens, which carry HP0546-specific material labeled with 10-nm gold grains at polar (top) and lateral (bottom) localizations, respectively, are shown. The bottom right panel depicts an inset at a higher magnification. Arrows point to flagella. Black bars are size markers of 0.5 μm.
The affinity-purified antiserum raised against a surface-exposed epitope of the putative pilin HP0546 does not inhibit IL-8 release by infected epithelial cells.
We next sought to determine whether the anti-HP0546 peptide antiserum was able to interfere with the functionality of the cag apparatus, of which HP0546 is predicted to form an important external part. In a preincubation step, the antiserum and negative control sera (dilution, 1:50 or 1:100) were added to HP88-3887 bacteria in cell culture medium supplemented with 10% FBS for 1 h and then supplemented at the same concentration during a 4-h or overnight coincubation of gastric epithelial cells with the bacteria. The affinity-purified antiserum under these conditions did not change the outcome of the bacterial coincubation with AGS cells (IL-8 release, hummingbird phenotype [not shown]). Preincubation with the anti-HP0546 peptide antiserum did not reduce the viability of the bacteria, which was confirmed by plating and counting of CFU after incubation for 1 h in the presence or absence of the peptide antiserum (not shown).
DISCUSSION
We described and partially characterized the HP0546 protein, encoded on the cag pathogenicity island of H. pylori, which has similarity to VirB2-like pilins of other T4SS (9, 26, 27). HP0546 is surface exposed, strain variable, and predominantly localized in the bacterial membrane fraction. The hypothesis that HP0546 may be a VirB2 pilin ortholog was also raised by a different group during the course of the present experiments in a review article (21), based on protein and gene synteny comparisons. The HP0546 gene was present in all H. pylori strains with an intact and functional cag PAI. The strain-to-strain variability of the HP0546-derived proteins was significant and localized (i) to the N terminus (putative leader peptide), (ii) to the domain adjacent to the postulated cleavage site of the leader peptide, and (iii) to the extreme C terminus. An antiserum raised against the N-terminal domain of HP0546 adjacent to the putative leader peptide cleavage site (TSPAEGVTETKTLVIQ) proved to be strain specific for the strain (26695) against which it was raised and recognized a small protein of approximately 10 kDa, which was absent from the HP0546 mutant. The protein was predominantly localized in the membrane fractions and to a lesser extent on bacterial surfaces. Immunofluorescence and electron microscopy with intact nonpermeabilized bacteria suggested that HP0546 subunits form or are part of a multimeric structure on bacterial surfaces. Expression and surface localization of native HP0546-containing material in individual H. pylori bacteria were not uniform. In electron microscopy specimens, the specific antiserum against HP0546 revealed surface-exposed material with no fixed location on bacterial cells and no defined morphology or length, which clearly exceeded the expected dimensions of monomeric HP0546. The possibility that the lack of defined structure may be due to preparation artifacts cannot be excluded. Structure will be studied further, with employment of different preparation techniques. We identified one second strain (M49), harboring a HP0546 sequence identical to that for 26695, in a collection of diverse H. pylori isolates from all over the world (13) (B. Linz, unpublished data), but this strain carried a nonfunctional cag PAI and did not react with the specific serum, suggesting that the protein is not expressed in this strain. We additionally determined the expression and localization of HP0546 in an isogenic cag virB4 (HP0544) mutant, which shows a functional defect of the cag system (15), and whose VirB4 ortholog in A. tumefaciens is required for T-pilus assembly (48). HP0546 was expressed in the HP0544 mutant but appeared to lose surface localization, indicating that its surface exposure is dependent on the cag T4SS. Secretion, surface expression, and processing of HP0546 in various cag mutants will be examined in more detail in further experiments.
We were able to express HP0546 as an internal FLAG tag fusion protein in both E. coli and A. tumefaciens, both of which can form functional T4SS with roles in DNA and protein transport. In both heterologous hosts, even in the absence of a functional T4SS, the HP0546 protein localized mostly to the insoluble fraction, presumably the membrane. These results support the concept that the expression and membrane localization of this protein are quite likely not dependent on a fully functional type IV apparatus and that membrane insertion may be determined by the general secretory pathway (32, 36), similar to evidence obtained for A. tumefaciens VirB2 (9, 25, 48). The detection of at least two bands in all bacterial species analyzed for the expression of the FLAG-tagged HP0546 using an anti-FLAG tag antibody indicated that the protein is processed. The detection of only one band (the one of lower mass) in Western blots using the specific anti-HP0546 peptide antiserum may suggest that the epitope (part of the synthesized peptide) against which the antiserum is directed is masked in the unprocessed form of the protein by the leader peptide. The HP0546 protein was detected as three bands in A. tumefaciens NT1REB. A. tumefaciens VirB2 and orthologous E. coli TrBCRP4 are cyclized after the cleavage of the leader peptide, by covalent linkage between the processed N and C termini (11, 21, 26). Cyclized A. tumefaciens VirB2 migrates slightly faster in SDS-PAGE than noncyclized VirB2 (25, 28). Partial cyclization of HP0546 in A. tumefaciens might explain the occurrence of three protein bands in Western blots. Two HP0546-specific bands were also detected when HP0546-FLAG was expressed in H. pylori, indicating that processing of HP0546 is not due to the heterologous expression systems but seems to occur also in H. pylori. Attempts to perform N-terminal protein sequencing of the HP0546 band detected by the specific anti-peptide antiserum were not successful (not shown). This may be due to cyclization of the mature protein, similar to that of A. tumefaciens VirB2 or to other ways of N-terminal processing. The slight elevation of the molecular mass of the surface-associated native HP0546 in H. pylori 88-3887 over those of its membrane-bound and cytoplasmic forms was also indicative of a further processing of the mature surface-associated HP0546 in H. pylori. The nature of this processing or posttranslational modification could not be determined here and will be the subject of further studies, including the analysis of various H. pylori cag mutants and the application of mass spectrometry.
The microscopic visualization of material on bacterial surfaces using the strain-specific anti-H. pylori HP0546 peptide antiserum provided evidence that the peptide used for the generation of the antiserum likely comprises a surface-exposed part of the mature pilin ortholog, probably within a multimeric assembly, and that it is not included in the predicted leader peptide. The amino acid epitope reacting with the strain-specific antibody could be narrowed down tentatively to the sequence SPAEGVT (aa 32 to 38 of predicted HP0546), since this motif varied and was different from that in HP0546 of strain 26695 in all other (except one) of the 42 tested strains (see Fig. S1 in the supplemental material). This result indicated that the sequence SPAEGVT is surface exposed in the mature structure formed by HP0546 subunits. A high selective pressure for variation on this surface-localized epitope may explain the high strain-to-strain variation of its amino acid sequence, leading to its absolutely strain-dependent recognition by the specific antiserum. The high variability of this surface-localized epitope of HP0546 in different strains may provide host specificity for interaction with a receptor and/or a means of antigenic variation.
Surface localization of the HP0546 protein was dependent on the cag system and appeared increased upon contact with eukaryotic cells, although the expression level of the protein seemed largely independent of the presence of human host cells. The surface appendage containing HP0546 subunits may form a functional pilus of the cag T4SS, promoting host interaction, although our structural characterization has not revealed a defined pilus structure so far. In recent publications, cag PAI-associated, pilus-like structures, containing three cag PAI-encoded proteins, HP0527, HP0528, and HP0532, which bear only limited similarity to the HP0546-containing material identified in the present study, were visualized on bacterial surfaces after cell contact (33, 43). Further studies will have to clarify whether and how both of these structural findings can be reconciled. In confirmation of previous results, HP0546 deletion mutants displayed diminished abilities to induce IL-8 release in gastric epithelial cells in comparison to the wild-type strain carrying an intact cag PAI (15, 38). The mutants also lost the ability to translocate the effector protein CagA into host cells (not shown; see also reference 31). The HP0546 protein appeared not to be strongly involved in bacterial adhesion to human gastric epithelial cells, since the isogenic mutants did not display deficiencies in adherence assays (our unpublished results). The specific antiserum directed against an apparently surface-exposed epitope of HP0546 did not inhibit the function of the cag PAI T4SS in coculture experiments with AGS gastric epithelial cells in our assays. However, since HP0546 is surface associated and very likely one of the proteins localized at the outermost edge of the cag PAI apparatus, which may have the ability to interact directly with epithelial cell receptors, the further characterization of this protein and its potential human receptors bears potential for the development of a vaccine or a specific therapeutic agent against cag-positive strains of H. pylori, which have been shown to be more pathogenic and have a higher association with ulcers and cancer in infected humans (1, 17, 40, 46).
. .
Supplementary Material
Acknowledgments
We thank Allison Stack, Verena Ryan, Daniela Fischer, and Daniela Goeppel for excellent technical assistance, all lab members for critical comments and helpful suggestions, and Matthias Frosch for continuous support. We are grateful to P. T. Hajdukiewicz and the CAMBIA Intellectual Property Resource (Canberra, Australia) for the kind gift of plasmid pCAMBIA-0380.
This work was supported by the German Research Council (grant Jo 344/2-1 and International Research Training Group grant IRTG 587/II to S. K. Lee) and by the PathoGenoMik Research Center Wuerzburg of the German Ministry of Education and Research (BMBF).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. [DOI] [PubMed] [Google Scholar]
- 2.Alm, R. A., L.-S. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 3.Backert, S., E. Ziska, V. Brinkmann, U. Zimny-Arndt, A. Fauconnier, P. R. Jungblut, M. Naumann, and T. F. Meyer. 2000. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2:155-164. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, S., T. Kwok, R. Hartig, W. Konig, and S. Backert. 2005. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 102:9300-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buhrdorf, R., C. Forster, R. Haas, and W. Fischer. 2003. Topological analysis of a putative virB8 homologue essential for the cag type IV secretion system in Helicobacter pylori. Int. J. Med. Microbiol. 293:213-217. [DOI] [PubMed] [Google Scholar]
- 6.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesnokova, O., J. B. Coutinho, I. H. Khan, M. S. Mikhail, and C. I. Kado. 1997. Characterization of flagella genes of Agrobacterium tumefaciens, and the effect of a bald strain on virulence. Mol. Microbiol. 23:579-590. [DOI] [PubMed] [Google Scholar]
- 8.Christie, P. J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churin, Y., L. Al Ghoul, O. Kepp, T. F. Meyer, W. Birchmeier, and M. Naumann. 2003. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 161:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbrandt, R., M. Kalkum, E. M. Lai, R. Lurz, C. I. Kado, and E. Lanka. 1999. Conjugative pili of IncP plasmids, and the Ti plasmid T pilus are composed of cyclic subunits. J. Biol. Chem. 274:22548-22555. [DOI] [PubMed] [Google Scholar]
- 12.Engelman, D. M., T. A. Steitz, and A. Goldman. 1986. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Biophys. Chem. 15:321-353. [DOI] [PubMed] [Google Scholar]
- 13.Falush, D., T. Wirth, B. Linz, J. K. Pritchard, M. Stephens, M. Kidd, M. J. Blaser, D. Y. Graham, S. Vacher, G. I. Perez-Perez, Y. Yamaoka, F. Megraud, K. Otto, U. Reichard, E. Katzowitsch, X. Wang, M. Achtman, and S. Suerbaum. 2003. Traces of human migrations in Helicobacter pylori populations. Science 299:1582-1585. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero, R. L., V. Cussac, P. Courcoux, and A. Labigne. 1992. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J. Bacteriol. 174:4212-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 16.Hajdukiewicz, P., Z. Svab, and P. Maliga. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25:989-994. [DOI] [PubMed] [Google Scholar]
- 17.Hatakeyama, M. 2004. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4:688-694. [DOI] [PubMed] [Google Scholar]
- 18.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257:519-528. [DOI] [PubMed] [Google Scholar]
- 19.Jin, Q., and S. Y. He. 2001. Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae. Science 294:2556-2558. [DOI] [PubMed] [Google Scholar]
- 20.Josenhans, C., K. A. Eaton, T. Thevenot, and S. Suerbaum. 2000. Switching of flagellar motility in Helicobacter pylori by reversible length variation of a short homopolymeric sequence repeat in fliP, a gene encoding a basal body protein. Infect. Immun. 68:4598-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkum, M., R. Eisenbrandt, R. Lurz, and E. Lanka. 2002. Tying rings for sex. Trends Microbiol. 10:382-387. [DOI] [PubMed] [Google Scholar]
- 22.Krall, L., U. Wiedemann, G. Unsin, S. Weiss, N. Domke, and C. Baron. 2002. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:11405-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 170:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai, E. M., O. Chesnokova, L. M. Banta, and C. I. Kado. 2000. Genetic and environmental factors affecting T-pilin export and T-pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J. Bacteriol. 182:3705-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai, E. M., R. Eisenbrandt, M. Kalkum, E. Lanka, and C. I. Kado. 2002. Biogenesis of T pili in Agrobacterium tumefaciens requires precise VirB2 propilin cleavage and cyclization. J. Bacteriol. 184:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai, E. M., and C. I. Kado. 1998. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 180:2711-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai, E. M., and C. I. Kado. 2000. The T-pilus of Agrobacterium tumefaciens. Trends Microbiol. 8:361-369. [DOI] [PubMed] [Google Scholar]
- 28.Lai, E. M., and C. I. Kado. 2002. The Agrobacterium tumefaciens T pilus composed of cyclic T pilin is highly resilient to extreme environments. FEMS Microbiol. Lett. 210:111-114. [DOI] [PubMed] [Google Scholar]
- 29.Manchak, J., K. G. Anthony, and L. S. Frost. 2002. Mutational analysis of F-pilin reveals domains for pilus assembly, phage infection and DNA transfer. Mol. Microbiol. 43:195-205. [DOI] [PubMed] [Google Scholar]
- 30.Moese, S., M. Selbach, T. Kwok, V. Brinkmann, W. Konig, T. F. Meyer, and S. Backert. 2004. Helicobacter pylori induces AGS cell motility and elongation via independent signaling pathways. Infect. Immun. 72:3646-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odenbreit, S., J. Püls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 32.Peabody, C. R., Y. J. Chung, M. R. Yen, D. Vidal-Ingigliardi, A. P. Pugsley, and M. H. Saier, Jr. 2003. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 149:3051-3072. [DOI] [PubMed] [Google Scholar]
- 33.Rohde, M., J. Puls, R. Buhrdorf, W. Fischer, and R. Haas. 2003. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol. Microbiol. 49:219-234. [DOI] [PubMed] [Google Scholar]
- 34.Sagulenko, E., V. Sagulenko, J. Chen, and P. J. Christie. 2001. Role of Agrobacterium VirB11 ATPase in T-pilus assembly and substrate selection. J. Bacteriol. 183:5813-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Sauvonnet, N., G. Vignon, A. P. Pugsley, and P. Gounon. 2000. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 19:2221-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimoyama, T., and J. E. Crabtree. 1997. Mucosal chemokines in Helicobacter pylori infection. J. Physiol. Pharmacol. 48:315-323. [PubMed] [Google Scholar]
- 40.Shimoyama, T., and J. E. Crabtree. 1998. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut 43(Suppl. 1):S2-S5. [PMC free article] [PubMed] [Google Scholar]
- 41.Suerbaum, S., J. Maynard Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA. 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka, J., T. Suzuki, H. Mimuro, and C. Sasakawa. 2003. Structural definition on the surface of Helicobacter pylori type IV secretion apparatus. Cell. Microbiol. 5:395-404. [DOI] [PubMed] [Google Scholar]
- 44.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 45.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 46.Webb, P. M., J. E. Crabtree, D. Forman, et al. 1999. Gastric cancer, cytotoxin-associated gene A-positive Helicobacter pylori, and serum pepsinogens: an international study. Gastroenterology 116:269-276. [DOI] [PubMed] [Google Scholar]
- 47.Yamada, H., T. Aihara, and S. Okabe. 2001. Mechanism for Helicobacter pylori stimulation of interleukin-8 production in a gastric epithelial cell line (MKN 28): roles of mitogen-activated protein kinase and interleukin-1beta. Biochem. Pharmacol. 61:1595-1604. [DOI] [PubMed] [Google Scholar]
- 48.Yuan, Q., A. Carle, C. Gao, D. Sivanesan, K. A. Aly, C. Hoppner, L. Krall, N. Domke, and C. Baron. 2005. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J. Biol. Chem. 280:26349-26359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.