Abstract
Although opportunistic infections like cytomegalovirus (CMV) are common sequelae of end-stage AIDS, the immune events leading to CMV reactivation in human immunodeficiency virus (HIV)-infected individuals are not well defined. The role of cellular and humoral CMV-specific immune responses in immune control of latent CMV infection was evaluated prospectively in a cohort of 11 simian immunodeficiency virus (SIV)-infected CMV-seropositive rhesus macaques, 6 of whom had histologic evidence of CMV disease at death. Macaques with CMV disease differed from macaques without CMV disease in having significantly higher levels of plasma SIV RNA and CMV DNA and significantly lower titers of anti-CMV binding antibodies (Abs) at the time of death. A significant decline in anti-CMV Abs and CMV-specific CD4+ and CD8+ T lymphocytes over time was observed in the macaques with CMV disease, but not in the macaques without CMV disease. Reduction in CMV-specific CD8+ T lymphocytes and anti-CMV neutralizing Abs was significantly correlated with a decline in CMV-specific CD4+ T lymphocytes. Although declines in CMV-specific T lymphocytes alone were sufficient for reactivation of low-level CMV viremia, high-level viremia (>1,000 copies of CMV DNA per ml of plasma) was observed when anti-CMV neutralizing and binding Abs had also declined. Thus, the occurrence of CMV reactivation-associated disease in AIDS is associated with suppression of both cellular and humoral CMV-specific immune responses. The underlying mechanism may be a dysfunction of memory B and CD8+ T lymphocytes associated with SIV-induced impairment of CMV-specific CD4+ T-cell help.
Although the use of highly active antiretroviral therapy (HAART) has led to a considerable decline in the incidence of opportunistic infections in AIDS, nonresponding individuals with advanced human immunodeficiency virus (HIV) infection continue to be at risk for developing cytomegalovirus (CMV) disease (13). In HIV-infected individuals, CMV seropositivity, primary CMV coinfection, and CMV viremia can be independent risk factors for accelerated progression to AIDS (24, 25, 30). AIDS-related CMV disease is usually a result of reactivation of preexistent, latent CMV infection. In HIV infection, CMV disease usually manifests after peripheral CD4+ T-lymphocyte counts have dropped below 100 cells per μl (8). Plasma HIV and CMV loads have also been shown to be independent risk factors predictive of occurrence of CMV disease in HIV-infected individuals (30).
In humans, the role of host immunity in control of CMV replication and prevention of CMV end-organ disease has been largely studied in immunosuppressed bone marrow or stem cell transplant recipients. These studies have established a central role for CMV-specific CD8+ and CD4+ T lymphocytes in resolution of CMV viremia and recovery from CMV disease (7, 31). Although high titers of neutralizing antibodies (Abs) have been correlated with the absence of plasma CMV DNA and improved survival following CMV disease (26), the relative contributions of CMV-specific Abs and cell-mediated immune responses to protection against CMV reactivation in humans are not known.
The reduced incidence of CMV disease following HAART-induced immune reconstitution and the association between regression of CMV disease and recovery of CMV-specific CD4+ T lymphocytes suggest that pathogen-specific immunity is important for containment of CMV replication in HIV-infected individuals (16). However, the precise immune correlates that protect against CMV reactivation in AIDS have not been characterized. Monitoring the correlates of protective CMV-specific immune responses may serve as an early predictive marker for identifying individuals at high risk for CMV disease prior to the detection of increased CMV viremia. Furthermore, therapeutic strategies that boost protective immune responses to CMV, and thereby limit CMV viremia in AIDS, are also likely to have a beneficial effect on the outcome of HIV infection.
We have used the simian immunodeficiency virus (SIV)-rhesus macaque model to prospectively investigate viral and immunologic risk factors associated with CMV reactivation in AIDS. CMV disease has been reported in up to 30% of rhesus macaques with simian AIDS (4). The similarities between simian and human CMV infection with regard to natural history, immune responses, and disease progression (14, 15, 28) make the rhesus macaque a valuable model for the study of CMV pathogenesis in AIDS. A significant advantage over human studies is the relative ease of conducting prospective studies and the ability to longitudinally evaluate changes in CMV-specific immune responses before and after pathogenic lentiviral infection. In the present study, we have investigated the temporal relationship between suppression of CMV-specific cellular and humoral immunity and occurrence of CMV end-organ disease in a cohort of CMV-seropositive rhesus macaques monitored from the time of SIV inoculation until the onset of AIDS.
MATERIALS AND METHODS
Animals and virus inoculation.
Rhesus macaques with naturally acquired CMV infection housed at the New England Primate Research Center were identified by serologic screening and enrolled in the study. Animals were housed in compliance with federal and institutional guidelines for animal care (2).
Eleven CMV-seropositive juvenile macaques were inoculated with 27 ng of the p27 equivalent of pathogenic SIVmac251 intravenously and monitored longitudinally. Elective euthanasia was performed by standard criteria after the onset of end-stage AIDS. Blood was collected once a week for the first 4 weeks after SIV inoculation and thereafter at biweekly to bimonthly intervals until time of death.
Vaccinia virus recombinants.
Plasmids containing molecularly cloned fragments of the rhesus CMV (rhCMV) strain 68-1 genome were used as templates for amplification of immediate-early protein 1 (IE1) exon 4, IE2 exon 5 (3), and the phosphoprotein pp65 (E. L. Blewett, R. H. Kravitz and P. Barry, unpublished observations). Amplification of the rhCMV interleukin-10 (IL-10) coding region used a plasmid containing a cDNA copy of IL-10 as the template (18). Amplicons were cloned into the TOPO-TA cloning vector (Invitrogen, Carlsbad, Calif.) and sequenced to confirm fidelity of amplification. The open reading frames were subcloned into the vaccinia virus recombination vector pAbT 4587 (Therion Biologic Corporation, Cambridge, Mass.) by using appropriate restriction sites, and the insertion sites of the recombinant plasmids were sequenced to confirm fidelity of cloning.
In each recombinant vaccinia virus, cDNAs encoding the rhCMV proteins IE1, IE2, pp65, and IL-10 were put under the control of the vaccinia virus early/late 40K promoter (9). The foreign genes were inserted into the HindIII M region of the genome of the NYCBH strain of vaccinia virus. Recombinant vaccinia viruses containing IE1, IE2, pp65, or IL-10, and designated rV-rhCMVIE1, rV-rhCMVIE2, rV-rhCMVpp65, and rV-rhCMVIL-10, respectively, were selected and purified by using a host-range selection system as previously described (19).
Immunophenotyping.
Fluorochrome-conjugated antihuman monoclonal Abs (MAbs) used for flow cytometry included CD4 allophycocyanin (clone SK3; BD Biosciences, San Diego, Calif.), CD3 phycoerythrin or fluorescein isothiocyanate (FITC; clone SP34; BD Biosciences), and CD8 peridinin chlorophyll protein (clone SK1; BD Biosciences). Flow samples were run on a FACSCalibur fluorescence-activated cell sorter (FACS) (BD Biosciences) and analyzed with Cellquest software.
Measurement of CMV-specific CD4+ T lymphocytes.
Peripheral blood CMV-specific CD4+ T lymphocytes were quantitated by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays with whole rhCMV antigen for stimulation as previously described (15). Spots were counted with a KS ELISPOT automated reader system (Carl Zeiss, Inc., Thornwood, N.Y.) using KS ELISPOT 4.2 software (performed by Zellnet, New York, N.Y.) and expressed as the number of spot-forming cells (SFC) per million CD4+ T lymphocytes.
Measurement of CMV-specific CD8+ T lymphocytes.
Peripheral blood CMV-specific CD8+ T lymphocytes responding to rhCMV proteins were quantitated by the IFN-γ ELISPOT assay with vaccinia virus recombinants for in vitro stimulation. By intracellular cytokine staining, we have shown that stimulation with vaccinia virus recombinants leads to cytokine secretion by CD3+ CD8+ T lymphocytes but not by CD4+ T lymphocytes (A. Kaur, unpublished data). Peripheral blood mononuclear cell (PBMC) aliquots were adsorbed for 90 min at 37°C with rV-rhCMVIE1, rV-rhCMVIE2, rV-rhCMVpp65, rV-rhCMVIL-10, or the control vaccinia virus NYCBH strain at a multiplicity of infection of 3 PFU per cell. Infected cells were washed and placed overnight in IFN-γ-coated ELISPOT wells. The frequency of CMV-specific CD8+ T lymphocytes was expressed as the sum of specific responses to individual rhCMV IE1, IE2, pp65 and IL-10 proteins and reported as the number of SFC per million PBMCs.
Neutralizing Abs to rhCMV.
Serial serum dilutions were incubated with gradient-purified rhCMV strain 68-1 for 4 h at 37°C. Viral titers were adjusted to give 100 to 150 infected cell counts at a magnification of ×200 magnification on a fluorescence microscope (Olympus/IMT-2), this being equivalent to 2,000 infected cells out of 15,000 cells. Fibroblasts (1.5 × 104) were added to the serum-virus mix and plated on microtiter plates. rhCMV-infected cells were counted 16 h later by indirect immunofluorescence with a rabbit serum directed against the IE protein of rhCMV (3). The percent neutralization was calculated as the reciprocal of infectivity, with maximum infectivity being determined by incubation of virus without serum. The rhCMV neutralizing Ab titer conferring 50% neutralization was reported.
Binding antibodies to rhCMV, rhesus LCV, and RRV.
Abs binding to whole rhCMV antigen, whole rhesus rhadinovirus (RRV) antigen, and a synthetic peptide derived from the rhesus lymphocryptovirus (LCV) small viral capsid antigen rhBFRF3 were detected by enzyme-linked immunosorbent assay (ELISA) as previously described (6, 15, 23). All binding Ab titers were determined by end-point dilution titration. Negative cutoff values were set at three standard deviations above the mean absorbance value obtained either with negative control serum tested at the lowest dilution or from triplicate wells that contained secondary Ab but no sera.
Plasma CMV DNA.
Plasma CMV DNA was quantitated by real-time PCR as previously described (15). CMV DNA was quantitated with a plasmid standard containing the entire rhCMV IE gene. This assay detects rhCMV with a linear dynamic range from 100 to 106 copies in the presence of genomic DNA.
Plasma SIV RNA.
Blood samples were collected in tubes containing EDTA, which were spun at 1,200 × g for 10 min within 3 h of drawing blood and stored at −80°C for quantitation of SIV RNA. SIV RNA was quantitated with a real-time reverse transcription (RT)-PCR assay as previously described (17).
Diagnosis of CMV disease.
All SIV-infected macaques were monitored up to the natural onset of AIDS. At death, all animals underwent necropsy and a comprehensive evaluation of multiple tissues. CMV end-organ disease was diagnosed by histopathologic examination of tissues. In all instances, the diagnostic histologic triad of cytomegalic cells, amphophilic intranuclear inclusion bodies, and neutrophilic infiltrate was confirmed to be of CMV origin by immunohistochemistry with a polyclonal rabbit Ab to the rhCMV IE1 protein (3).
Statistical analysis.
Statistical analysis was carried out with Statview (Abacus Concepts, Inc., Berkeley, Calif.). P values for differences between groups were determined by the Mann-Whitney U test and for differences between time points by the Wilcoxon signed rank test. The relationship between two continuous variables was analyzed by the Spearman rank correlation test. The relationship between two nominal variables was compared with the Fisher exact probability test.
RESULTS
CMV disease in SIV-infected rhesus macaques.
Among the 11 CMV-seropositive rhesus macaques monitored longitudinally after SIV inoculation, 6 macaques had histopathological evidence of CMV disease at necropsy; AIDS-defining illnesses other than CMV were detected in the remaining five macaques (Table 1). Rhesus macaques with and without CMV disease had similar ages at the time of SIV inoculation (Table 1). In contrast to macaques without CMV disease, macaques with CMV disease had a significantly shorter survival time (Table 1); two of six died within 12 weeks of SIV inoculation, and 5 of 6 did not mount a detectable Ab response to SIV (data not shown). The tissue sites with CMV end-organ disease included the lung (n = 2), lung and testes (n = 1), stomach (n = 1), jejunum (n = 1), and lymph node (n = 1).
TABLE 1.
Characteristics of the cohort of SIV-infected chesus macaques with and without CMV disease
| CMV end-organ disease at deatha | No. of animals | Age (yr) at SIV infection
|
Time (wk) to AIDS
|
AIDS-defining illness other than CMV disease (no. present)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Pnemocystis carinii pneumonia | Disseminated Mycobacterium avium | SIV encephalitis | Bacterial colitis | Protozoan gastroenteritis | Other infectionsb | ||
| Present | 6 | 3.9 | 2.3-4.6 | 17 | 10-30 | 2 | 1 | 4 | 0 | 2 | 3 |
| Absent | 5 | 3.8 | 2.2-5.1 | 57 | 2 | 2 | 1 | 2 | 0 | 0 | |
| P valuec | 0.93 | 0.01 | |||||||||
Diagnosis of CMV end-organ disease made by histology.
One macaque each with cryptosporidium hepatitis, SV40 nephritis, and adenovirus infection in the jejunum.
Mann-Whitney U test.
Increased CMV load and high levels of SIV viremia are predictive of CMV disease in SIV-infected rhesus macaques with AIDS.
In HIV-infected humans, severe CD4+ T lymphocytopenia (CD4 counts of <100/μl) has consistently been shown to be a risk factor for occurrence of CMV disease in subjects with AIDS (8). In addition, high HIV load and high CMV load are predictive of development of CMV disease in HIV-infected humans (13, 30). We investigated whether similar risk factors predisposed to the occurrence of CMV disease in the cohort of SIV-infected rhesus macaques.
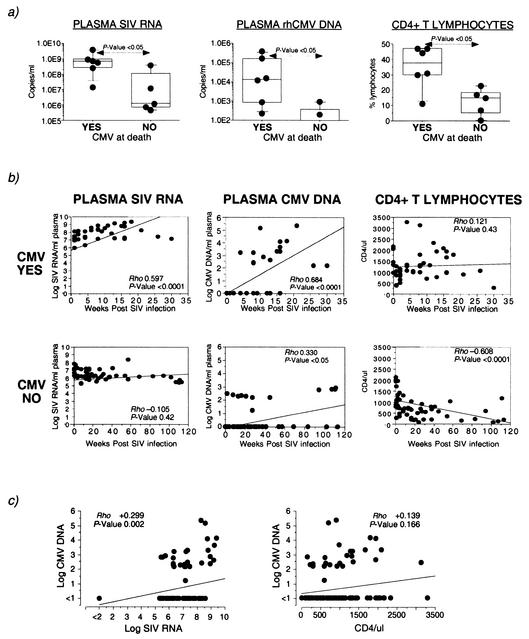
At the time of death, the median plasma SIV RNA level in macaques with CMV disease (6.4 × 108 copies per ml) was 2 logs higher than that in macaques without CMV disease (median, 1.2 × 106 copies per ml) (Fig. 1a). The difference in plasma SIV RNA levels between the two groups of macaques was apparent as early as 8 weeks postinfection (P < 0.05, Mann-Whitney U test). Furthermore, a significant increase in plasma SIV load with time was observed in the macaques with CMV disease, but not in the macaques without CMV disease (Fig. 1b).
FIG. 1.
Plasma SIV and CMV load and CD4+ T-lymphocyte counts in SIV-infected rhesus macaques with or without CMV disease. (a) Box plots comparing levels of plasma SIV RNA, plasma CMV DNA, and CD4+ T-lymphocyte counts at the time of death in six SIV-infected rhesus macaques with CMV disease (YES) and five SIV-infected rhesus macaques without CMV disease (NO). Horizontal bars show median values, boxes indicate 75% confidence intervals, and error bars show 95% confidence intervals. P values were determined by the nonparametric Mann-Whitney U test. (b) Correlation between time post-SIV infection and plasma SIV RNA, CMV DNA, and CD4+ T-lymphocyte counts. (c) Correlation between CMV load and SIV load or CD4+ T lymphocytes. Correlation coefficient (Rho) and P values were determined by the Spearman rank correlation coefficient test.
Plasma CMV DNA was detected at the time of death in six of six macaques with CMV disease, but in only two of five macaques without CMV disease (Fig. 1a). Using real-time PCR, we have not detected CMV DNA in the plasma of 36 SIV-negative CMV-seropositive rhesus macaques (Kaur, unpublished). Although plasma CMV DNA was detected at one or more time points in 10 of 11 rhesus macaques during the course of SIV infection, levels above 1,000 copies per ml were only seen in four of six macaques that progressed to CMV disease (Fig. 1a and b).
Contrary to the observed association between severe CD4+ T lymphocytopenia and increased risk of occurrence of CMV disease in HIV-infected humans, six of six SIV-infected macaques with CMV disease had CD4 counts of >200/μl at the time of death. Paradoxically, the peripheral CD4+ T-lymphocyte counts were significantly lower in the macaques that did not develop CMV disease (Fig. 1a), and a significant decline in peripheral CD4+ T-lymphocyte counts during the course of SIV infection was observed only in this group of macaques (Fig. 1b). Surprisingly, the difference in CD4+ T-lymphocyte counts between macaques with and without CMV disease was also apparent as early as 8 weeks post-SIV infection (P < 0.05, Mann-Whitney U test). Consistent with these data, the increase in CMV load was directly correlated with an increase in SIV load (P < 0.01), but not with a decrease in CD4+ T-lymphocyte counts (Fig. 1c).
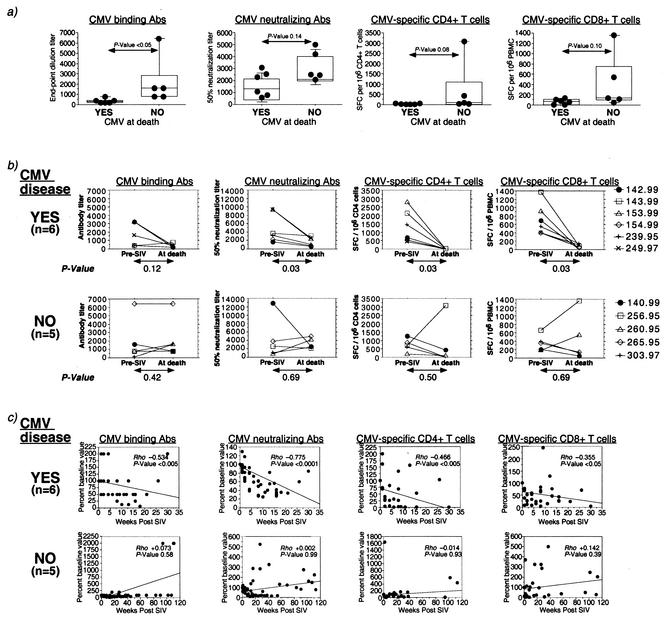
CMV disease in rhesus macaques with AIDS is associated with a sustained decline in both the humoral and cellular components of CMV-specific immunity.
At the time of death, macaques with CMV disease had significantly lower titers of anti-rhCMV binding Abs compared to the macaques without CMV disease (Fig. 2a and Table 2). There was a wide range in the magnitude of CMV-specific immune parameters pre- and post-SIV infection (Table 2). Macaques that progressed to CMV disease did not have lower levels of measurable CMV-specific immune responses pre-SIV infection (Table 2). Rather, baseline frequencies of CMV-specific CD8+ T lymphocytes were significantly higher in macaques with CMV disease (Table 2).
FIG. 2.
Magnitude and kinetics of decline of CMV-specific humoral and cellular immunity in SIV-infected rhesus macaques with or without CMV disease. (a) Box plots comparing CMV-specific binding and neutralizing Ab titers and the frequency of CMV-specific CD4+ and CD8+ T cells at the time of death in SIV-infected rhesus macaques with and without CMV disease. P values were determined by the nonparametric Mann-Whitney U test. Binding Ab titers were determined by end-point dilution. Neutralizing Ab titers refer to the reciprocal dilution inducing 50% neutralization. (b) Extent of decline in CMV-specific immune parameters during the course of SIV infection in individual animals. P values were determined by the nonparametric Wilcoxon signed rank test. (c) Changes in the level of CMV-specific immune parameters with time during the course of SIV infection. Rho and P values were determined by the Spearman rank correlation coefficient test.
TABLE 2.
Comparison of CMV-specific immune responses in SIV-infected rhesus macaques with and without CMV disease
| CMV disease (n) | Anti-CMV binding Ab titera
|
Anti-CMV 50% neutralizing Ab titer
|
CMV-specific CD4+ T cells (SFC/106 CD4+ cells)
|
CMV-specific CD8+ T cells (SFC/106 PBMCs)
|
||||
|---|---|---|---|---|---|---|---|---|
| Pre-SIV infectionc | At time of deathc | Pre-SIV infection | At time of death | Pre-SIV infection | At time of death | Pre-SIV infection | At time of death | |
| Present (6) | 1,000 (400-3,200) | 300 (200-800) | 3,425 (1,650-9,350) | 1,700 (600-3,100) | 1,054 (428-2,788) | 2 (0-18) | 617 (395-1,368) | 68 (0-134) |
| Absent (5) | 800 (80-6,400) | 1,600 (800-6,400) | 2,600 (800-12,800) | 2,500 (2,100-5,000) | 707 (187-1,258) | 93 (0-3,086) | 349 (188-665) | 133 (48-1,361) |
| P valueb | 0.93 | 0.01∗ | 0.58 | 0.14 | 0.47 | 0.08 | 0.03∗ | 0.10 |
End-point dilution titer.
Mann-Whitney U test comparing macaques with and without CMV disease. Asterisks indicate significant P values.
Median (range) values are shown.
While the absolute levels of neutralizing Abs or CMV-specific T lymphocytes at death were not predictive of CMV disease, a significant decline in the titer of neutralizing Abs and in the frequency of CMV-specific CD4+ and CD8+ T lymphocytes from baseline values was observed in the majority of macaques with CMV disease, but not in the macaques without CMV disease (Fig. 2b). On examining the longitudinal change in magnitude of immune parameters during the course of SIV infection, a significant, progressive decline in the frequency of CMV-specific CD4+ and CD8+ T lymphocytes, and in the titers of anti-CMV neutralizing and binding Abs was observed over time in the rhesus macaques with CMV disease, but not in the macaques without CMV disease (Fig. 2c).
Although CMV-specific CD4+ and CD8+ T lymphocytes had declined in the majority of SIV-infected rhesus macaques, regardless of the occurrence of CMV disease (Fig. 2b and Table 3), the mean decline in CMV-specific CD8+ T lymphocytes was >50-fold higher in the macaques with CMV disease than in the macaques without CMV disease (P < 0.05, Mann-Whitney U test). In contrast to the fairly ubiquitous decline in CMV-specific T lymphocytes in SIV-infected macaques, a twofold or greater decline in anti-CMV binding and neutralizing Abs was detected in five of six macaques with CMV disease but in only one of five macaques without CMV disease (P = 0.08, Fisher exact test; Table 3).
TABLE 3.
Comparison of declines in CMV-specific immune responses at the time of death in SIV-infected rhesus macaques with and without CMV disease
| CMV end-organ disease at death (n) | CMV binding Abs
|
CMV-neutralizing Abs
|
CMV-specific CD4+ T lymphocytes
|
CMV-specific CD8+ T lymphocytes
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. with decline | Fold decline | No. with decline | Fold decline | No. with decline | No. undetectable | Fold decline | No. with decline | No. undetectable | Fold decline | |
| Present (6) | 5/6 | 2-16 | 5/6 | 3-4 | 6/6 | 6/6 | 67-2,788 | 6/6 | 2/6 | 4-913 |
| Absent (5) | 1/5 | 2 | 1/5 | 5 | 4/5 | 2/5 | 2-865 | 3/5 | 0/5 | 3-4 |
| P valuea | 0.080 | 0.080 | 0.455 | 0.182 | ||||||
Fisher's exact probability test.
Temporal relationship between CMV viral load and CMV-specific immunity.
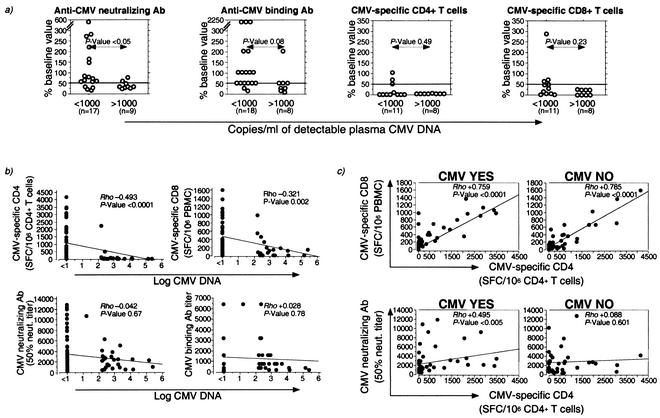
Since the appearance of CMV disease was associated with suppression of both humoral and cellular CMV-specific immune responses, we investigated the components of virus-specific immunity that had declined at time points with detectable CMV viremia. Plasma CMV DNA was detected at a total of 27 time points in 10 of 11 macaques during the course of SIV infection. Levels above 1,000 copies of CMV DNA per ml of plasma (high-level CMV viremia) were only observed in macaques that subsequently manifested CMV disease, while levels of <1,000 copies of CMV per ml of DNA (low-level CMV viremia) were detected in both groups of macaques. A decline in CMV-specific T lymphocytes was observed with comparable frequency at time points with less than or more than 1,000 copies of CMV DNA per ml of plasma (Fig. 3a and Table 4) and was sufficient for detection of low-level CMV viremia. In contrast, a decline in anti-CMV neutralizing Abs was observed at a significantly higher frequency at time points with high-level compared to low-level CMV viremia (Fig. 3a and Table 4). Similarly, a concurrent decline in anti-CMV Abs and CMV-specific T lymphocytes occurred at a significantly higher frequency at time points with high-level compared to low-level CMV viremia (Table 4).
FIG. 3.
Relationship between CMV load and CMV-specific humoral and cellular immune responses in SIV-infected rhesus macaques. (a) Relationship between decline in CMV-specific immune parameters and detection of <1,000 or >1,000 copies of detectable CMV DNA per ml of plasma. A cutoff value of 50% decline is demarcated on each plot. n denotes the number of time points for which a CMV-specific immune parameter measurement was available. The Fisher exact probability test was used for comparison of differences in frequency of decline between the two groups. (b) Relationship between plasma CMV DNA and the frequency of CMV-specific T lymphocytes or titers of anti-CMV Ab. (c) Relationship between CMV-specific CD4+ T lymphocytes and CMV-specific CD8+ T lymphocytes or anti-CMV neutralizing Ab. Rho and P values were determined by the Spearman rank correlation coefficient test.
TABLE 4.
Relationship between decline in CMV-specific immunity and CMV viremia
| No. of copies of plasma CMV DNA/ml | No. of time pointsa | No. (%) of time points with ≥50% decline in CMV-specific immune parameterb:
|
||||
|---|---|---|---|---|---|---|
| Neutraliz- ing Ab | Total Ab | CD4+ T cells | CD8+ T cells | All 4 parametersc | ||
| <1,000 | 11 | 2 (18.2) | 4 (36.4) | 9 (81.8) | 8 (72.7) | 2 (18.2) |
| >1,000 | 7 | 6 (85.7) | 6 (85.7) | 7 (100) | 7 (100) | 6 (85.7) |
| P valued | <0.05 | 0.07 | 0.50 | 0.25 | <0.05 | |
Time points at which all four CMV-specific immune parameters were measured.
Time points from 10 of 11 SIV-infected macaques with detectable CMV viremia.
Anti-CMV neutralizing Ab, anti-CMV total Ab, CMV-specific CD4+ T cells and CMV-specific CD8+ T cells.
Fisher's exact probability test.
In addition to CMV-specific CD4+ and CD8+ T lymphocytes, anti-CMV Ab had declined in all but one macaque with CMV disease at the time of death. Interestingly, plasma CMV DNA levels were low (231 copies per ml) in this animal, and only focal CMV disease in the jejunum was seen histologically. Overall, these data suggest that a relatively intact CMV-specific humoral response may limit CMV replication and dissemination in SIV-infected rhesus macaques.
Analysis of all animals revealed that plasma CMV viral load was inversely correlated with the frequency of CMV-specific CD4+ and CD8+ T lymphocytes (Fig. 3b). A weak inverse correlation between plasma CMV load and neutralizing Ab was observed in the macaques with CMV disease (Rho, −0.362; P = 0.02; Spearman rank correlation test). However, this correlation was not observed when all the animals were analyzed together (Fig. 3b) and is consistent with the preponderance of time points with low-level CMV viremia. The frequencies of CMV-specific CD4+ and CD8+ T lymphocytes were tightly correlated with each other irrespective of the presence or absence of CMV disease (Fig. 3c) (data not shown). On the other hand, a modest direct correlation between anti-CMV neutralizing Ab titers and the frequency of CMV-specific CD4+ T lymphocytes was only observed in the macaques with CMV disease (Fig. 3c). Although titers of anti-CMV neutralizing and binding Abs were directly linked with each other (Rho, +0.608; P < 0.0001; Spearman rank correlation test), we did not observe a correlation between the frequency of CMV-specific CD4+ T lymphocytes and anti-CMV binding Ab (data not shown).
Selective decline in anti-CMV Ab in rhesus macaques with CMV disease.
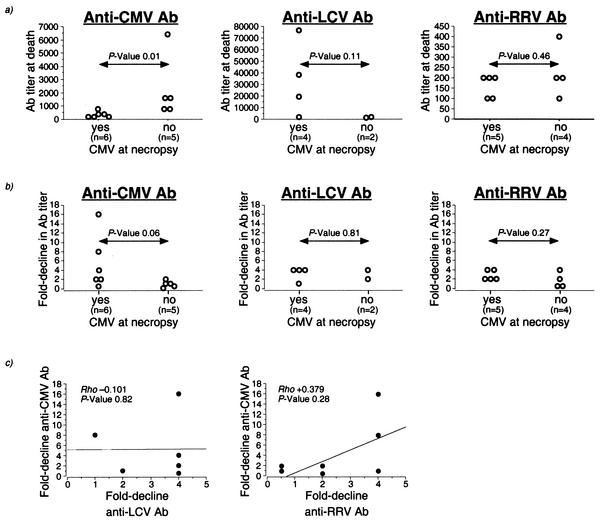
Since the majority of rhesus macaques with a significant decline in anti-CMV Abs were rapid progressors, we investigated whether this reflected a selective or global decline in memory humoral responses. In individual animals, we compared the decline in anti-CMV binding Ab titers to the decline in binding Ab titers to RRV and LCV, two herpesvirus infections that are widely prevalent among rhesus macaques (6, 20). At the time of death, contrary to anti-CMV Ab, anti-LCV and anti-RRV Ab were not present at lower levels in the macaques with CMV disease compared to the macaques without CMV disease (Fig. 4a). Although a decline in anti-LCV and anti-RRV Abs was seen in the majority of SIV-infected rhesus macaques tested, there was no difference in the extent of Ab decline between macaques with and without CMV disease (Fig. 4b). Finally, there was no correlation between the decline in anti-CMV and anti-LCV Abs (Fig. 4c). Thus, a macaque with a 16-fold decline in anti-CMV Ab had a fourfold decline in anti-LCV Ab, and a macaque with an eightfold decline in anti-CMV Ab had no decline in anti-LCV Ab (Fig. 4c). Although there was a trend towards a positive correlation between the decline in anti-CMV Ab and anti-RRV Ab, it did not reach statistical significance (Fig. 4c). Together, these data suggest that the decline in humoral immunity in macaques with CMV disease was virus specific.
FIG. 4.
Comparison of decline in anti-CMV, anti-LCV, and anti-RRV Abs in SIV-infected rhesus macaques with and without CMV disease. Anti-LCV Abs were measured in four macaques with CMV disease and two macaques without CMV disease. Anti-RRV Abs were measured in all study animals. Two macaques were RRV seronegative prior to SIV infection and were excluded from analysis. (a) Ab titers at time of death in macaques with (Yes) and without (No) CMV disease. P values were determined by Mann-Whitney U test. (b) Fold decline from pre-SIV infection values in anti-CMV, anti-LCV, and anti-RRV Abs in macaques with (Yes) and without (No) CMV disease. P values were determined by Mann-Whitney U test. (c) Relationship between decline in anti-CMV Ab and either anti-LCV Ab or anti-RRV Ab. Rho and P values were determined by the Spearman rank correlation coefficient test.
DISCUSSION
Although the link between immunosuppression and occurrence of CMV disease in AIDS is well established, to our knowledge this is the first report of a longitudinal and concurrent evaluation of the humoral and cellular components of CMV-specific immunity in relation to CMV reactivation in AIDS. In this study, we provide evidence for a complementary role between cellular and humoral immunity in the control of CMV replication in a cohort of 11 CMV-seropositive SIV-infected rhesus macaques. Occurrence of histologic CMV disease was associated with rapid disease progression, lower titers of anti-CMV binding Ab and higher SIV and CMV viral loads at death, and a significant decline over time in CMV-specific CD4+ and CD8+ T lymphocytes and anti-CMV neutralizing Abs.
Rather than absolute levels, we observed that the magnitude and rate of decline of CMV-specific Abs and CMV-specific CD4+ and CD8+ T lymphocytes clearly differentiated macaques that progressed to CMV disease from macaques that did not develop CMV disease. Increases in plasma CMV DNA associated with a 50% or greater decline in the frequency of CMV-specific CD4+ and CD8+ T lymphocytes were readily detected in SIV-infected macaques with or without CMV disease. However, levels above 1,000 copies per ml, seen only in four of six macaques with CMV disease, were detected when anti-CMV Ab had also declined to 50% or less of baseline values. In instances in which CMV disease manifested in the presence of a relatively intact CMV-specific humoral immune response (two of six macaques), plasma CMV DNA levels did not exceed 1,000 copies per ml, and histologic evidence of CMV disease was focal, suggesting a role for anti-CMV Ab in limiting viral dissemination. Previous studies on correlation between CMV disease and host immunity in humans and macaques with AIDS have been restricted to the analysis of a single component of CMV-specific immunity (1, 14-16, 27, 28). The contributions of different components of CMV-specific immunity in control of CMV replication have been elegantly studied in the murine CMV model. In one such study, CMV recurrence in B-cell-deficient mice did not occur with depletion of a single lymphocyte subset (22). Instead, depletion of CD8+ T cells in combination with NK and/or CD4+ T cells resulted in CMV recurrence at most tissue sites (22). Although an absence of virus-specific Abs in itself is not sufficient to induce CMV reactivation in murine CMV infection, the magnitude of recurrent viremia following T-cell suppression is 100- to 1,000-fold higher in B-cell-deficient as compared to normal mice (11). The correlation between decline in anti-CMV Ab and occurrence of disseminated CMV disease in our study is consistent with these findings.
In pathogenic lentiviral infection, loss of T helper cell function may be the primary factor leading to loss of virus-specific CD8+ T lymphocytes and T-dependent Ab production (10, 12). A strong positive correlation between the frequencies of CMV-specific CD4+ and CD8+ T lymphocytes was observed prior to SIV infection and persisted during the course of SIV infection in macaques with and without CMV disease, suggesting that the fates of these two cell populations were tightly linked. CMV-specific CD4+ T lymphocytes were also positively correlated with anti-CMV neutralizing Abs. However, this correlation was modest and only apparent in the SIV-infected macaques that developed CMV disease. It is not known to what extent the long-term maintenance of anti-CMV neutralizing Abs is dependent on long-lived plasma cells (29) or on memory B lymphocytes that continually differentiate into short-lived Ab-secreting plasma cells (21). Since loss of CD4 T-cell help has been shown to significantly reduce memory neutralizing antibodies by inhibiting differentiation of memory B cells (21), it is likely that a similar mechanism may be operating in SIV-infected macaques and HIV-infected humans.
A variety of B-cell abnormalities, including loss of memory B lymphocytes, have been reported in HIV-infected individuals (5). If the decline in anti-CMV Ab in SIV-infected macaques was due to a global loss of memory B cells, one would expect to observe a decline in other virus-specific Abs. This did not appear to be the case, as evidenced by the discordant decline in titers of Ab to CMV when compared with those to two other persistent herpesviruses. The relatively selective decline in anti-CMV Ab could also have resulted from binding of Ab to excess circulating virus and in itself does not imply a causal association with CMV disease. The observation of a relatively intact CMV humoral immune response in animals with no or focal CMV disease in our study and the occurrence of fulminant CMV infection in SIV-infected macaques with a poor primary CMV Ab response (28) suggest that Abs do have a role in immune control of CMV replication.
Similar to HIV-infected humans, high SIV and CMV viral loads were significant risk factors for development of CMV end-organ disease in SIV-infected rhesus macaques. However, in contrast to HIV infection, CD4+ T lymphocytopenia was not a prerequisite for occurrence of CMV end-organ disease. Neither did it appear to enhance the risk of developing CMV disease. In fact, CD4+ T-lymphocyte counts below 200/μl were only seen in two animals in this cohort, neither of which developed CMV end-organ disease. Since the majority of rhesus macaques with CMV disease progressed very rapidly to AIDS, it is possible that the absence of CD4+ T lymphocytopenia is a reflection of the short disease duration, rather than a species-specific difference. Since both rapid and normal progressors have been observed among SIV-infected macaques with histologic CMV disease (4), we cannot exclude the possibility of CMV disease in chronically SIV-infected macaques being more akin to CMV disease in HIV-infected humans.
Rapid disease progression is a common feature of primary CMV infection in SIV-infected macaques (28). In this study, all animals had acquired CMV infection naturally and were CMV seropositive for at least 2 years prior to SIV infection. The animals were individually housed from 3 weeks prior to SIV infection until the end of the study and hence were unlikely to have gotten superinfected with other strains of rhCMV following SIV infection. Whether the rapid disease progression was a cause or effect of CMV disease cannot be determined from this study.
In conclusion, our data provide evidence for a complex interaction between both humoral and cellular CMV-specific immune responses in preventing CMV reactivation in AIDS. Although the importance of CD4+ T-cell help in maintaining an effective antiviral CD8+ T-lymphocyte response is well established, our study also demonstrates a significant association between impaired CD4+ T-cell help and induction of memory B-cell dysfunction. Rather than absolute differences in the level of CMV-specific immune responses, both the magnitude and kinetics of decline in CMV-specific immune responses appeared to predispose SIV-infected macaques to development of CMV disease. A decline in a single immune parameter was not sufficient to induce CMV disease. In this cohort, a fourfold or greater decline in the frequency of CMV-specific CD4+ and CD8+ T lymphocytes combined with a twofold or greater decline in anti-CMV binding and neutralizing Abs was a harbinger of disseminated CMV disease.
Acknowledgments
This study was supported by Public Health Service grants RR00168, AI43890 (A.K.), and AI45314 (R.P.J.); federal funds from the National Cancer Institute, National Institutes of Health, under contract no. N01-CO-12400 (J.D.L.); and grant MA929/4-2 from the Deutsche Forschungsgemeinschaft (M.M.).
We gratefully acknowledge Barbara Kropff for the neutralization assays, Hannah Sanford for the RRV ELISA, Carol Quink for the LCV ELISA, Marie-Claire Gauduin for the SIVmac251 virus stock, Angela Carville and the staff of the Department of Primate Medicine for superb assistance with the animal experiments, and Martin Hirsch for critical review of the manuscript.
REFERENCES
- 1.Alberola, J., A. Tamarit, L. Cardenoso, F. Estelles, R. Igual, and D. Navarro. 2001. Longitudinal analysis of human cytomegalovirus glycoprotein B (gB)-specific and neutralizing antibodies in AIDS patients either with or without cytomegalovirus end-organ disease. J. Med. Virol. 64:35-41. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1996. Guide for the care and use of laboratory animals, p. 86-123. The Institute of Laboratory Animal Resources, National Research Council, Washington, D.C.
- 3.Barry, P. A., D. J. Alcendor, M. D. Power, H. Kerr, and P. A. Luciw. 1996. Nucleotide sequence and molecular analysis of the rhesus cytomegalovirus immediate-early gene and the UL121-117 open reading frames. Virology 215:61-72. [DOI] [PubMed] [Google Scholar]
- 4.Baskin, G. B., M. Murphey-Corb, E. A. Watson, and L. N. Martin. 1988. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet. Pathol. 25:456-467. [DOI] [PubMed] [Google Scholar]
- 5.De Milito, A., C. Morch, A. Sonnerborg, and F. Chiodi. 2001. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS 15:957-964. [DOI] [PubMed] [Google Scholar]
- 6.Desrosiers, R. C., V. G. Sasseville, S. C. Czajak, X. Zhang, K. G. Mansfield, A. Kaur, R. P. Johnson, A. A. Lackner, and J. U. Jung. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71:9764-9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einsele, H., E. Roosnek, N. Rufer, C. Sinzger, S. Riegler, J. Loffler, U. Grigoleit, A. Moris, H. G. Rammensee, L. Kanz, A. Kleihauer, F. Frank, G. Jahn, and H. Hebart. 2002. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99:3916-3922. [DOI] [PubMed] [Google Scholar]
- 8.Gallant, J. E., R. D. Moore, D. D. Richman, J. Keruly, and R. E. Chaisson. 1992. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J. Infect. Dis. 166:1223-1227. [DOI] [PubMed] [Google Scholar]
- 9.Gritz, L., A. Destree, N. Cormier, E. Day, V. Stallard, T. Caiazzo, G. Mazzara, and D. Panicali. 1990. Generation of hybrid genes and proteins by vaccinia virus-mediated recombination: application to human immunodeficiency virus type 1 env. J. Virol. 64:5948-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquot, S., T. Kobata, S. Iwata, C. Morimoto, and S. F. Schlossman. 1997. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J. Immunol. 159:2652-2657. [PubMed] [Google Scholar]
- 11.Jonjic, S., I. Pavic, B. Polic, I. Crnkovic, P. Lucin, and U. H. Koszinowski. 1994. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J. Exp. Med. 179:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan, J. E., D. L. Hanson, J. L. Jones, and M. S. Dworkin. 2001. Viral load as an independent risk factor for opportunistic infections in HIV-infected adults and adolescents. AIDS 15:1831-1836. [DOI] [PubMed] [Google Scholar]
- 14.Kaur, A., M. D. Daniel, D. Hempel, D. Lee-Parritz, M. S. Hirsch, and R. P. Johnson. 1996. Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian immunodeficiency virus-infected rhesus macaques. J. Virol. 70:7725-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur, A., C. L. Hale, B. Noren, N. Kassis, M. A. Simon, and R. P. Johnson. 2002. Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J. Virol. 76:3646-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komanduri, K. V., M. N. Viswanathan, E. D. Wieder, D. K. Schmidt, B. M. Bredt, M. A. Jacobson, and J. M. McCune. 1998. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat. Med. 4:953-956. [DOI] [PubMed] [Google Scholar]
- 17.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockridge, K. M., S. S. Zhou, R. H. Kravitz, J. L. Johnson, E. T. Sawai, E. L. Blewett, and P. A. Barry. 2000. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268:272-280. [DOI] [PubMed] [Google Scholar]
- 19.Mazzara, G. P., A. Destree, and A. Mahr. 1993. Generation and analysis of vaccinia virus recombinants. Methods Enzymol. 217:557-581. [DOI] [PubMed] [Google Scholar]
- 20.Moghaddam, A., M. Rosenzweig, D. Lee-Parritz, B. Annis, R. P. Johnson, and F. Wang. 1997. An animal model for acute and persistent Epstein-Barr virus infection. Science 276:2030-2033. [DOI] [PubMed] [Google Scholar]
- 21.Ochsenbein, A. F., D. D. Pinschewer, S. Sierro, E. Horvath, H. Hengartner, and R. M. Zinkernagel. 2000. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl. Acad. Sci. USA 97:13263-13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Luccaronin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao, P., H. Jiang, and F. Wang. 2000. Cloning of the rhesus lymphocryptovirus viral capsid antigen and Epstein-Barr virus-encoded small RNA homologues and use in diagnosis of acute and persistent infections. J. Clin. Microbiol. 38:3219-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robain, M., F. Boufassa, J. B. Hubert, A. Persoz, M. Burgard, and L. Meyer. 2001. Cytomegalovirus seroconversion as a cofactor for progression to AIDS. AIDS 15:251-256. [DOI] [PubMed] [Google Scholar]
- 25.Sabin, C. A., H. L. Devereux, G. Clewley, V. C. Emery, A. N. Phillips, C. Loveday, C. A. Lee, and P. D. Griffiths. 2000. Cytomegalovirus seropositivity and human immunodeficiency virus type 1 RNA levels in individuals with hemophilia. J. Infect. Dis. 181:1800-1803. [DOI] [PubMed] [Google Scholar]
- 26.Schoppel, K., C. Schmidt, H. Einsele, H. Hebart, and M. Mach. 1998. Kinetics of the antibody response against human cytomegalovirus-specific proteins in allogeneic bone marrow transplant recipients. J. Infect. Dis. 178:1233-1243. [DOI] [PubMed] [Google Scholar]
- 27.Schrier, R. D., C. A. Wiley, C. Spina, J. A. McCutchan, and I. Grant. 1996. Pathogenic and protective correlates of T cell proliferation in AIDS. J. Clin. Investig. 98:731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sequar, G., W. J. Britt, F. D. Lakeman, K. M. Lockridge, R. P. Tarara, D. R. Canfield, S.-S. Zhou, M. B. Gardner, and P. A. Barry. 2002. Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis. J. Virol. 76:7661-7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slifka, M. K., R. Antia, J. K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363-372. [DOI] [PubMed] [Google Scholar]
- 30.Spector, S. A., K. Hsia, M. Crager, M. Pilcher, S. Cabral, and M. J. Stempien. 1999. Cytomegalovirus (CMV) DNA load is an independent predictor of CMV disease and survival in advanced AIDS. J. Virol. 73:7027-7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]