Abstract
Skeletal muscle mitochondria are implicated with age-related loss of function and insulin resistance. We examined the effects of exercise on skeletal muscle mitochondria in older (age=67.3±0.6 yr) men (n=5) and women (n=3). Similar increases in (P<0.01) cardiolipin (88.2±9.0 to 130.6±7.5 μg/mU creatine kinase activity (CK)) and the total mitochondrial DNA (1264±170 to 1895±273 copies per diploid of nuclear genome) reflected increased mitochondria content. Succinate oxidase activity, complexes 2–4 of the electron transport chain (ETC), increased from 0.13±0.02 to 0.20±0.02 U/mU CK (P<0.01). This improvement was more pronounced (P<0.05) in SS (127±48%) compared to IMF (56±12%) mitochondria. NADH oxidase activity, representing total ETC activity, increased from 0.51±0.09 to 1.00±0.09 U/mU CK (P<0.01). In conclusion, exercise enhances mitochondria ETC activity in older human skeletal muscle, particularly in SS mitochondria, which is likely related to the concomitant increases in mitochondria biogenesis.
INTRODUCTION
Aging has been associated with a reduced capacity for oxidative phosphorylation in muscle (1) (2), most likely due to a decline in mitochondria content and/or function (3). A poor capacity for oxidative metabolism within skeletal muscle is also associated with insulin resistance (4) and type 2 diabetes mellitus (5; 6). Recent studies further indicate that muscle mitochondria of patients with type 2 diabetes are smaller, less numerous and may also be less functional than those without diabetes (7). Petersen et al. have further suggested that a lower oxidative capacity in muscle is an essential feature of age-associated insulin resistance (8). However, it is not clear from these cross-sectional studies whether this lower oxidative capacity of muscle may be due to deficiencies in mitochondria content, a reduced mitochondria function, or both. It also raises the important question of whether mitochondria defects observed in normal aging and in metabolic disorders are the result of an acquired problem, and accordingly, whether they can be restored with intervention. While it is known that young healthy muscle is quite plastic in its ability to increase its capacity for oxidative metabolism in response to chronic exercise, less is known whether muscle in pathophysiological conditions, or even muscle of healthy older adults is able to respond accordingly to intervention.
Given the strong evidence linking mitochondria dysfunction with aging, insulin resistance and type 2 diabetes, it is important to more precisely define specific loci of these defects, and perhaps more importantly, to determine whether clinical interventions may correct these insufficiencies. Previous studies have demonstrated the presence of two distinct mitochondrial populations within skeletal muscle (9–11). Sub-sarcolemmal (SS) mitochondria reside near the sarcolemma, and inter-myofibrillar (IMF) mitochondria are located between the myofibrils. It has been suggested that SS mitochondria provide energy for membrane related events including cell signaling, substrate and ion transport, while IMF mitochondria supply ATP to contracting myofibrils (12). SS mitochondria generally represents only 25–30% of the total amount of skeletal muscle mitochondria but appear to be more responsive to increased physical activity in rat muscle (10; 13) as well as in young human skeletal muscle (14–16). Whether there are improvements in specific mitochondrial subpopulations in older adults with reduced mitochondria content and function has yet to be determined.
These prior observations question whether there are specific populations of mitochondria that may be more responsive to intervention, and moreover, whether improvements are simply due to increased mitochondrial content. In the present study we examined in older adults the effects of moderate exercise training on changes in mitochondria content and function located within distinct locations within skeletal muscle.
METHODS
Subjects
Eight healthy elderly (67.3 ± 0.6 yr) volunteers (3 women and 5 men) were recruited using community advertisements and participated in this study after providing written informed consent. None of the volunteers were currently engaged in regular (> 1 x/week) exercise, nor had they gained or lost more than 2 kg body weight within the past 6 months prior to the study. None of the volunteers had type 2 diabetes. Those with coronary heart disease, peripheral vascular disease, untreated hypertension or clinically significant hyperlipidemia (plasma triglycerides greater than 3.95 mmol/L or total cholesterol levels greater than 7.76 mmol/L) were excluded. The research project was reviewed and approved by the University of Pittsburgh Institutional Review Board.
Intervention
Subjects completed a 12-week exercise-training program, which has been previously described in detail for a similar group of older adults (17), and will be summarized briefly. Participants were asked to complete a minimum of 4 and a maximum of 6 exercise sessions weekly, with at least three supervised sessions weekly. Exercise was performed mostly using treadmills, stationary bicycles or outdoor walking, was individually prescribed based on time and intensity, and was progressive. For the first 4 weeks, they exercised for 30 minutes at a heart rate corresponding to 50–60% of maximal aerobic capacity (VO2max). For the next 4 weeks, they increased exercise time to 40 minutes at the same intensity, and for the last 4 weeks they increased the intensity to ~70% of VO2max for at least 40 minutes per session.
Study Protocol
Before and after 12 weeks of exercise, subjects had a percutaneous muscle biopsy, a test for physical fitness (maximal aerobic capacity; VO2max), and a blood sample during fasting conditions to determine markers of insulin resistance (glucose and insulin).
Maximal Aerobic capacity (VO2max)
Subjects performed a graded exercise test on an electronically-braked cycle ergometer (SensorMedics Ergoline 800S, Yorba Linda, CA) to determine changes in physical fitness (VO2max). Expired air was collected via open-circuit spirometry (SensorMedics 2900, Yorba Linda, CA) to determine VO2 and VCO2. Heart rate, blood pressure and ECG were recorded prior to, during and immediately following this test. Heart rate-VO2 relationships obtained during this graded exercise test were also used to prescribe intensity during each exercise training session.
Insulin Resistance
In order to determine the training effects on insulin resistance, we calculated homeostasis model assessment of insulin resistance (HOMA-IR), based on fasting glucose and insulin. Plasma glucose was measured using an automated glucose oxidase reaction (YSI 2300 Glucose Analyzer, Yellow Springs, OH). Serum insulin was determined using commercially available radioimmunoassay kits (Pharmacia, Uppsala, Sweden).
Muscle biopsies
Percutaneous biopsies of the vastus lateralis were obtained in the General Clinical Research Center (GCRC) on a morning after an overnight fast as described previously in more detail (17; 18). Subjects were given a standard 10 kcal/kg meal consisting of 50% carbohydrate, 30% fat, and 20% protein the night before the biopsy. Subjects were instructed not to perform physical exercise 48 h before the muscle biopsy procedure to help prevent acute effects of exercise on muscle mitochondria function. Muscle specimens were trimmed, frozen in liquid nitrogen and stored at −80° C. Baseline and post-intervention biopsy specimens from each subject were prepared and analyzed together to avoid any inter-assay variability in isolation of mitochondria or biochemical analysis.
Preparation of Mitochondrial Fractions
A portion of muscle biopsy samples of (~10–15 mg wet weight) were homogenized in ice-cold basic medium (100 mM mannitol 80 mM gluconate – K; 20 mM KF, 1 mM MgCl2, 0.2 mM EGTA, 10 mM histidine, 10 mM glucose, 10 mM TEA-MOPSO, pH 7.6 at 21°C) containing 5.0 mg/ml BSA, 100 μM deferoxamine mesylate, and anti-protease cocktail III, using a Polytron homogenizer according to the procedures described by Krieger et al. (10). All procedures were performed at 4°C. Soluble and particulate fractions were prepared as previously described (19), by centrifugation (45,000g for 20 min) to pellet a particulate (SS+IMF mitochondria) fraction containing > 95% of tissue mitochondria. SS and IMF mitochondria fractions were prepared as described earlier (20). SS mitochondria were isolated from skeletal muscle following gentle extraction procedures, and after the subsequent extraction of myosin, IMF mitochondria fraction was collected in two sub-fractions, a free fraction (IMF1) and another fraction more tightly bound to myofibrils (IMF2). Mitochondrial preparations were suspended in 500 μl of medium II, containing 0.5 mM EGTA, 0.1 mg/ml BSA, 25 mM KH2PO4, pH 7.0 at 21°C, and kept at −80°C until assay.
Mitochondria DNA Determinations
DNA (mitochondrial and nuclear) was extracted from tissue samples using a QIAamp DNA mini kit (QIAGEN, Chatworth, CA). The concentration of each sample was determined using a GeneQuant spectrophotometer (Pharmacia Biotech). Mitochondria DNA (MtDNA) content was measured using the real-time PCR as described earlier (20; 21). Detection of a 69 bp fragment of mtDNA (nucleotides 14918–14986) and a 77 bp fragment of β-globin, both based on markers published by Miller et al. (22), were used to determine relative copy number of mtDNA per diploid nuclear genome. Primers and FAM-labelled Taqman TAMRA probes (Applied Biosystems, # 450025) were designed using Primer Express software, version 1.5 (Applied Biosystems, Foster City, CA). Detection of mtDNA and β-globin was performed as two separate reactions but within the same run for each sample. All samples were run in duplicate for each gene. Reactions were carried out in the presence of 1X Taqman Universal PCR Master Mix (# 4304437, Applied Biosystems, Foster City, CA 94404-1128), 1 μM each forward and reverse primer, 0.25 μM (FAM) labeled Taqman/TAMRA probe, and 20 ng sample DNA to a final volume of 25 μl. Amplification reactions were performed in an ABI Prism 7700 spectrofluorometric thermal cycler (Applied Biosystems, Foster City, CA) with the following cycle conditions: 50°C for 2 min UNG incubation, 95°C denaturation and enzyme activation step for 10 min followed by 40 cycles of 95°C denaturation for 15 s, and 60°C annealing and elongation for 60 s. Fluorescence spectra were recorded during the annealing phase of each PCR cycle. The Sequence Detection System software (SDS v1.7) of the ABI-Prism 7700 was used to generate the FAM fluorescence.
Threshold cycle calculations
The threshold cycle number (Ct) was calculated using SDS software v1.7 and an automatic setting of the baseline. The baseline value was the average fluorescence value of PCR cycles 3–15 plus 10 times its standard deviation. These values were used for the relative copy number (Rc) calculations by expressing Ct differences of the β-globin and mtDNA PCR as described earlier (21):
Cardiolipin
Cardiolipin is a phospholipid specific to mitochondria, thus reflecting mitochondria content. Cardiolipin was quantified in each mitochondria sub-fraction of previously frozen skeletal muscle biopsies by high performance liquid chromatography analysis of a fluorescence-labeled derivative of cardiolipin (2-[naphthyl-1′-acetyl]-cardiolipin dimethyl ester) (23). Cardiolipin content was normalized to the amount of CK activity as the amount of active skeletal muscle.
Electron transport chain (ETC) Activity
Activity of NADH oxidase (rotenone-sensitive NADH:O2 oxidoreductase) was determined in total mitochondria fractions by an HPLC-based assay, as described previously to represent total (complexes I–IV) ETC activity (24; 25). Succinate oxidase (succinate: O2 oxidoreductase) activity was measured in total mitochondria fractions and separately in each SS, IMF1 and IMF2 mitochondria sub-fraction according to the separation scheme outlined above. Succinate oxidase is a reaction starting from succinate dehydrogenase (Complex II), and is based on assay of the accumulation of fumarate, the end-product of succinate oxidation as described earlier (20). This procedure is a modification of a previously developed assay (7; 26). Briefly, the assay couples fumarase and malic dehydrogenase reactions to oxidize fumarate and reduce NAD, with HPLC and fluorescence detection used to measure NADH (19). Activity of creatine kinase (CK) was measured an index of muscle fiber content in biopsy samples as previously described (7; 20; 25), and ETC activity is expressed normalized to CK activity.
Statistics
Data are presented as mean ± SEM, unless otherwise indicated. Paired t-tests were used to determine effects of exercise intervention on changes in mitochondria content, ETC activity, physical fitness and markers of insulin resistance. Two-way analysis of variance was used to compare sub-fractions of mitochondria and their differential response over time (mitochondria sub-fraction X time).
RESULTS
Body composition, physical fitness and insulin sensitivity
Body composition, physical fitness and markers of insulin resistance before and after the intervention are shown in Table 1. At baseline, participants were overweight but not obese, and were sedentary. The intervention improved physical fitness (VO2max) significantly (p<0.01) by 15 ± 4% and without a change in body weight or % body fat. There was a significant (p<0.05) improvement in insulin sensitivity estimated by HOMA IR, reflected mainly by a decrease in fasting plasma insulin (Table 1).
Table 1.
General body composition, physical fitness and markers of insulin resistance before and after the 12-week exercise program.
| PRE-TRAINING | POST-TRAINING | |
|---|---|---|
| BMI (kg/m2) | 28.0 ± 1.6 | 27.7 ± 1.5 |
| % body fat | 32.2 ± 2.2 | 32.4 ± 2.3 |
| VO2max (L/min) | 1.64 ± 0.14 | 1.88 ± 0.15* |
| Fasting glucose (mM) | 5.20 ± 0.13 | 5.12 ± 0.16 |
| Fasting Insulin (μU/ml) | 13.0 ± 2.6 | 10.2 ± 1.7* |
| HOMA-IR | 3.05 ± 0.63 | 2.32 ± 0.40* |
Results presented as means ± standard error of the mean; N=8 (3 women, 5 men); BMI, body mass index; HOMA-IR, homeostatic model estimate of insulin resistance; * denotes significant change from pre to post intervention, P<0.05 (paired t-test).
Mitochondria content
Skeletal muscle mitochondria DNA (mtDNA) content was determined in biopsy samples before and after the intervention, and the results are shown in Figure 1. At baseline, muscle mtDNA was lower in these elderly men and women in comparison to younger adults recently reported in our laboratory (24), which is also consistent with prior studies (3). Changes in mtDNA reflect changes in mitochondria volume. As illustrated in Figure 1, there was a robust increase (53 ± 15%) in mtDNA content with training (P<0.01). Cardiolipin, a mitochondria-specific phospholipid that reflects mitochondria content, increased by 56 ± 13% (Figure 2), corresponding to the increase in total mitochondria content assessed by mtDNA.
Figure 1. Effects of exercise on skeletal muscle mitochondria DNA content.
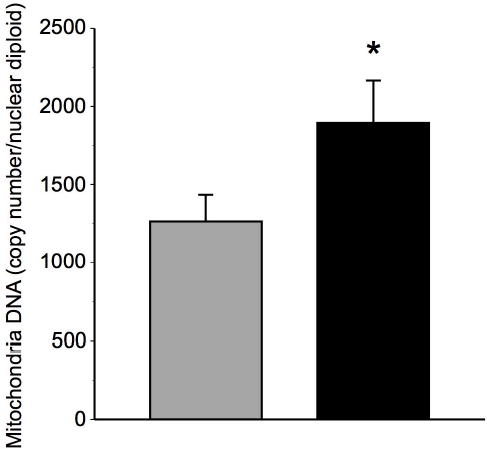
mtDNA copy number in total mitochondria fraction relative to diploid nuclear genome before (gray bars) and after (black bars) exercise training; * denotes significant (P<0.01) change determined by paired t-test; n=7; data are presented as means ± standard error of the mean.
Figure 2. Effects of exercise on skeletal muscle cardiolipin content in sub-sarcolemmal (SS) and inter-myofibrillar (IMF1 and IMF2) mitochondrial fractions.
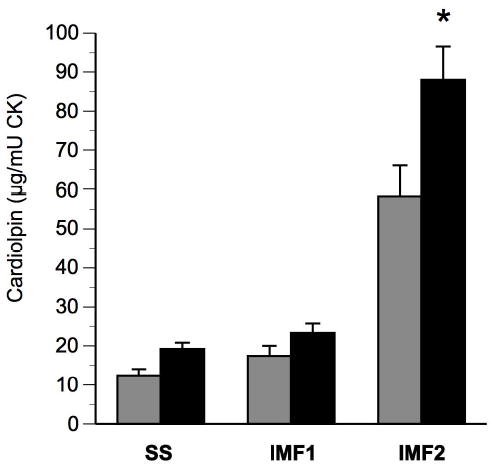
Cardiolipin content before (gray bars) and after (black bars) exercise training; * denotes significant (P<0.01) change determined by paired t-test; n=8; data are presented as means ± standard error of the mean; CK, enzymatic activity of skeletal muscle creatine kinase.
Mitochondrial ETC activity
Cardiolipin and mtDNa provide complimentary assessments of mitochondria content. To further assess the potential effect of exercise training, electron transport chain (ETC) activity, a functional measure, was assessed; specifically that of NADH oxidase and succinate oxidase, which represents mitochondria ETC activity from Complexes I to IV and II to IV, respectively. In response to intervention, activity of NADH oxidase in the total mitochondria fraction was approximately doubled (Figure 3). Succinate oxidase in the total mitochondria fraction increased (p<0.01) by 62 ± 13% (Figure 3) from 0.13 ± 0.02 to 0.20 ± 0.02 U·mU CK−1. The magnitude of these increases corresponded with the increase in the content of both total mtDNA and cardiolipin. This suggests that the increased mitochondrial content corresponded with the overall increase in mitochondria function in skeletal muscle.
Figure 3. Effects of exercise on total mitochondrial electron transport chain activity.
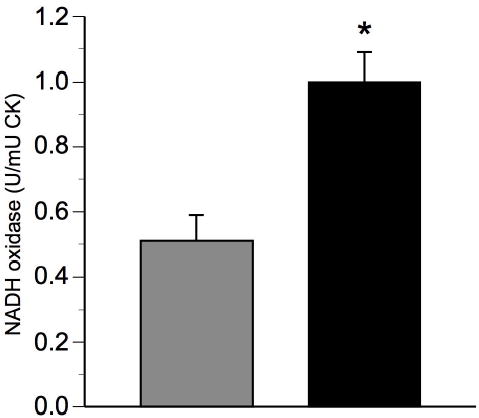
Total skeletal muscle NADH oxidase activity before (gray bars) and after (black bars) exercise training; Data are presented as means ± standard error of the mean; n=5; CK, activity of creatine kinase; * denotes significant change from Pre-intervention assessed by paired t-test, P<0.01.
Cellular Distribution of Mitochondria
As described in the Methods, mitochondria were isolated into three fractions, and cardiolipin content and ETC activity were assessed in each fraction. The results are shown in Figures 2 and 4. At baseline, the IMF2 fraction, which are those most tightly bound to myofibrils, contained the majority of cardiolipin (~65%), while the SS fraction contained a much smaller proportion of cardiolipin (~14%). Following intervention, there was a highly significant increase in cardiolipin content in the IMF2 and SS fractions. Following intervention, the relative proportion of cardiolipin content among the three remained essentially unchanged.
Figure 4.
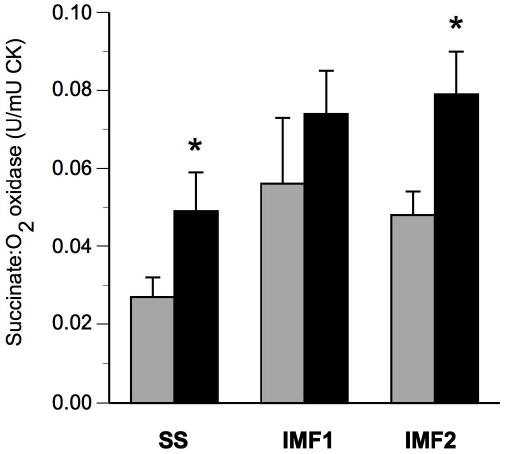
Effects of exercise on electron transport chain activity in sub-sarcolemmal (SS) and inter-myofibrillar (IMF1 and IMF2) mitochondrial fractions. Skeletal muscle succinate oxidase activity in SS and IMF fractions of skeletal muscle before (gray bars) and after (black bars) exercise. Data are presented as means ± standard error of the mean; n=8; SS; CK, activity of creatine kinase; * denotes significant change from Pre-intervention assessed by paired t-test, P<0.01.
ETC (succinate oxidase) activity contained within the SS and IMF fractions are shown in Figure 4. Prior to intervention, the distribution of ETC activity was not symmetric, such that there was a relative deficiency in the SS fraction (21 ± 3% of total ETC). Exercise training increased (P<0.01) the ETC activity in both SS and IMF2 mitochondria fractions of skeletal muscle (Figure 4). This increase in ETC activity was more pronounced in SS (127 ± 48%) than in IMF2 (65 ± 14%) mitochondria (p<0.05). There was not a statistically significant increase of ETC activity in the IMF1 fraction.
DISCUSSION
Impaired oxidative phosphorylation by skeletal muscle mitochondria have been postulated to contribute to age-associated insulin resistance and fat accumulation within skeletal muscle (8). This impaired mitochondrial functional capacity in aging has been attributed to a reduced mitochondrial content, as reflected by lower mtDNA content (3). The current study was therefore undertaken to assess the impact of physical activity upon muscle mitochondria in elderly men and women. As reflected in three independent and complementary parameters, mtDNA, cardiolipin and ETC activity, there was a substantial response of mitochondria, improvements of at least 50% in each of these parameters. These improvements were further assessed within distinct muscle mitochondria sub-populations. At baseline, a relatively low fraction of ETC activity and cardiolipin was contained in the sub-sarcolemmal (SS) fraction in these elderly volunteers, a finding that is entirely consistent with their overweight and sedentary status (24). In response to training, there was a robust improvement in the SS fraction, but there was also a substantial improvement in the inter-myofibrillar (IMF2) fraction, the sub-population of mitochondria that most directly provide energy for contracting muscle. Thus, our main finding is that there is a robust improvement in skeletal muscle mitochondrial content and function in elderly men and women in response to an achievable program of moderate intensity physical activity.
One of the classic responses to exercise is an increase in the oxidative capacity of skeletal muscle (27–29). Relatively few of the many human studies of exercise intervention, however, have focused upon the elderly. A few earlier studies have demonstrated that chronic endurance training increased the amount of mitochondrial protein (30) and mitochondrial volume (31) in skeletal muscle older men and women concomitant with enhanced overall physical fitness. In the current study, we have expanded upon this important earlier work by broadening the scope of biochemical assessments of mitochondrial content and function in response to increased physical activity in older men and women.
While the response observed in the participants of the current study was quite positive, it was not clear that this would in fact occur. Many age-related declines in physiological function can be partially attributed to mitochondria dysfunction (32; 33). There is a significant loss in the number of muscle fibers and also biochemical and morphological abnormalities in aging skeletal muscle (34; 35). The specific mechanisms leading to the age-related changes are currently unknown. Mitochondria are primary sites of reactive oxygen species formation that causes progressive damage to mitochondrial DNA and proteins (35; 36). The analysis of human muscle mitochondria has revealed a progressive decline in mitochondrial respiratory chain function with age (1; 3; 37; 38), which may be related to reduced mitochondria DNA content (3). These studies collectively raise the question of whether age-related mitochondrial defects are the result of normal aging or conversely, whether they are at least partially acquired through lifestyle and factors other than aging per se.
An important area for investigation is to more fully evaluate whether aging limits or alters the response of mitochondria to intervention. Previously, our laboratory (7; 20) has observed an impaired bioenergetic capacity of skeletal muscle mitochondria in type 2 diabetes and obesity, including smaller mitochondria and reduced electron transport chain (ETC) activity (7). The ETC activity in the healthy older participants in this study at baseline was 3-fold less than that observed for younger lean individuals but similar to that seen in middle-aged obese participants without type 2 diabetes (20). In particular, the lower ETC activity in these older men and women was pronounced in SS mitochondria compared to IMF mitochondria; there was approximately a 4-fold lower succinate oxidase activity in the SS mitochondria in these elderly participants in comparison with lean younger individuals (24). Similarly, Ritov et al. reported a greater deficiency in the SS mitochondria in type 2 diabetes and obesity (20). In contrast, the ETC activity in skeletal muscle of younger individuals was distributed evenly across the fractions with approximately one-third of overall activity in the SS fraction compared to 21% in these older adults (20). This suggests that there may be age-related reductions in oxidative capacity of muscle, and specifically, deficiencies in certain mitochondria sub-populations in aging. Alternatively, mitochondria sub-populations could be affected differently by physical activity related to aging. Although the response of these generally healthy older volunteers was quite robust, the limited sample size of our study prevents us from generalizing these results to older persons who may have functional impairments, more severe insulin resistance or type 2 diabetes.
Few studies have examined whether improvements in mitochondria function and/or content are related to the improvements in insulin resistance and risk for the development of metabolic syndrome or type 2 diabetes. HOMA-IR as a marker of insulin sensitivity (39) improved in parallel with improved mitochondria content and function. These results are consistent with the observations that higher oxidative capacity is related to higher insulin sensitivity (40) and that an exercise-enhanced reliance on fat oxidation predicts improved insulin sensitivity in obesity (41) and in the elderly (42). These results, however, are in apparent contrast to the study by Short et al. (43), who reported increased oxidative capacity in older men and women despite little improvement in insulin sensitivity. Thus, the relationship between increased mitochondria function and insulin resistance should be examined further.
This is the first study examining the effects of exercise on the function of distinct mitochondria sub-populations within aging skeletal muscle. The increase in ETC activity of complex II–IV (succinate oxidase) was more pronounced in SS mitochondria than in IMF mitochondria. The total ETC assessed by NADH oxidase activity, as well as total succinate oxidase activity, paralleled the increase in total mitochondrial content measured as mtDNA content and cardiolipin, all increasing by more than 50%. The degree of response in cardiolipin within the SS and IMF mitochondrial sub-fraction was closely matched to the increases in ETC within the same fraction. Moreover, the ratio of ETC activity to mitochondria content (cardiolipin) did not change with intervention. Previous studies have demonstrated that the SS mitochondria population is higher in young endurance-trained individuals to a greater extent than was the IMF population compared to untrained participants (29). Chilibeck et al. reported that endurance training resulted in greater increases in SS succinate dehydrogenase (SDH) activity compared to IMF mitochondria (15). However, Krieger et al. (10) reported similar increases in SDH activities in the SS and IMF mitochondria populations of rat skeletal muscle in response to chronic endurance training. SS mitochondria likely provide energy for cellular processes of substrate transport and cell signaling in skeletal muscle (12), and exhibit higher rates of fatty acid oxidation (44). Thus, SS mitochondria may be specifically linked to physical inactivity, low oxidative capacity and insulin resistance. Further inquiry into the functional significance of how different mitochondrial subpopulations in human skeletal muscle respond to various interventions might provide insight into mitochondria as potential therapeutic targets for prevention and treatment of insulin resistance and type 2 diabetes.
In summary, mitochondria function, as assessed by electron transport chain activity, and content of mitochondria improved similarly with exercise training. However, there were distinct differences in the response of electron transport chain activity within specific populations of skeletal muscle mitochondria. Additional studies are clearly needed to determine the specific function of these mitochondrial sub-populations, and further, to investigate whether there are specific components of mitochondria function that are implicated in age- and obesity-associated disorders. This could have implications for designing specific interventions, including exercise, in the treatment and prevention of skeletal muscle functional changes with aging.
Acknowledgments
We greatly appreciate the nursing staff of the University of Pittsburgh General Clinical Research Center and also Andreas Katsiaras and Donna Wolf for their efforts in carrying out the exercise intervention. We would like to express our appreciation to the research volunteers who participated in these studies. This work was funded by K01-AG-00851 and R01 AG20128-01 (BHG) and also the Obesity Nutrition Research (1P30DK46204) and General Clinical Research (5M01RR00056) Centers.
References
- 1.Cooper JM, Mann VM, Shapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992;113:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- 2.Coggan AR, Spina RJ, King DS, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Geront. 1992;47:B71–76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 3.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9:273–278. [PubMed] [Google Scholar]
- 5.Mootha V, CM L, Eriksson K-F, et al. PGC-1alpha responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genetics. 2003. [DOI] [PubMed]
- 6.Patti M, Butte A, Crunkhorn S, et al. Coordinated reduction in genes of oxidative metabolism in humans with insulin resistance and diabetes: potential roles of PGC1 and NRF-1. Proceedings of the National Academy of Science USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 8.Petersen K, Befoy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogswell AM, Stevens RJ, Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol (Endocrinol. Metab) 1993;264:C383–C389. doi: 10.1152/ajpcell.1993.264.2.C383. [DOI] [PubMed] [Google Scholar]
- 10.Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol. 1980;48:23–28. doi: 10.1152/jappl.1980.48.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Palmer JTB, Hoppel C. Biochemical Properties of Subsarcolemmal and Interfibrillar Mitochondria Isolated from Rat Cardiac Muscle. The Journal of Biological Chemistry. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 12.Hood DA. Invited Review: Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 13.Bizeau M, Willis W, Hazel J. Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J Appl Physiol. 1998;85:1279–1284. doi: 10.1152/jappl.1998.85.4.1279. [DOI] [PubMed] [Google Scholar]
- 14.Elander A, Sjostrom M, Lundgren F, Schersten T, Bylund-Fellenius AC. Biochemical and morphometric properties of mitochondrial populations in human muscle fibers. Clinical Science. 1985;69:153–164. doi: 10.1042/cs0690153. [DOI] [PubMed] [Google Scholar]
- 15.Chilibeck PD, Bell GJ, Socha T, Martin T. The effect of aerobic exercise training on the distribution of succinate dehydrogenase activity throughout muscle fibers. Can. J. Appl. Physiol. 1998;23:74–86. doi: 10.1139/h98-005. [DOI] [PubMed] [Google Scholar]
- 16.Chilibeck PD, Syrotuik DG, Bell GJ. The effect of concurrent endurance and strength training on quantitative estimates of subsarcolemmal and intermyofibrillar mitochondria. Int. J. Sports Med. 2002;23:33–39. doi: 10.1055/s-2002-19269. [DOI] [PubMed] [Google Scholar]
- 17.Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287:E857–E862. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- 18.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- 19.Ritov V, Kelley D. Hexokinase isozyme distribution in human skeletal muscle. Diabetes. 2001;50:1253–1262. doi: 10.2337/diabetes.50.6.1253. [DOI] [PubMed] [Google Scholar]
- 20.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of Sub-Sarcolemmal Mitochondria In Obesity and Type 2 Diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Szuhai K, van den Ouweland JM, Dirks RW, et al. Simultaneous A8344G heteroplasmy and mitochondrial DNA copy number quantification in Myoclonus Epilepsy and Ragged-Red Fibers (MERRF) syndrome by a multiplex Molecular Beacon based real-time fluorescence PCR. Nucleic Acids Research. 2001;29:e13. doi: 10.1093/nar/29.3.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller F, Rosenfeldt F, Zhang C, Linnane A, Nagley P. Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of change of copy number with age. Nucleic Acids Research. 2003;31:e61–e68. doi: 10.1093/nar/gng060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlame M, Shanske S, Doty S, et al. Microanalysis of cardiolipin in small biopsies including skeletal muscle from patients with mitochondrial disease. J Lipid Res. 1999;40:1585–1592. [PubMed] [Google Scholar]
- 24.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. American Journal of Physiology Endocrinology & Metabolism. 2005; 288. [DOI] [PubMed]
- 25.Ritov V, Menshikova E, Kelley D. High performance liquid chromatography-based methods of enzymatic analysis: electron transport chain activity in mitochondria from human skeletal muscle. Analytical Biochemistry. 2004;333:27–38. doi: 10.1016/j.ab.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Gostimskaya I, Grivennikova V, Zharova T, Bakeeva L, Vinogradov A. In situ assay of the intramitochondrial enzymes: use of alamethicin for permeabilization of mitochondria. Analytical Biochemistry. 2003;313:46–52. doi: 10.1016/s0003-2697(02)00534-1. [DOI] [PubMed] [Google Scholar]
- 27.Dohm GL, Huston RL, Askew EW, Fleshood HL. Effects of exercise, training, and diet on muscle citric acid cycle enzyme activity. Can J Biochem. 1973;51:849–854. doi: 10.1139/o73-105. [DOI] [PubMed] [Google Scholar]
- 28.Holloszy JO, Oscai LB, Don IJ, Mole PA. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–1373. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- 29.Hoppeler H, Luthi P, Claassen H, Weibel ER, Howald H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pfluegers Arch. 1973;344:217–232. doi: 10.1007/BF00588462. [DOI] [PubMed] [Google Scholar]
- 30.Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 31.Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. Journal of Applied Physiology. 2001;90:1663–1670. doi: 10.1152/jappl.2001.90.5.1663. [DOI] [PubMed] [Google Scholar]
- 32.Brierley EJ, Johnson MA, James OF, Turnbull DM. Effects of physical activity and age on mitochondrial function. Qjm. 1996;89:251–258. doi: 10.1093/qjmed/89.4.251. [DOI] [PubMed] [Google Scholar]
- 33.Hunter GR, Newcomer BR, Weinsier RL, et al. Age is independently related to muscle metabolic capacity in premenopausal women. Journal of Applied Physiology. 2002;93:70–76. doi: 10.1152/japplphysiol.01239.2001. [DOI] [PubMed] [Google Scholar]
- 34.Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Review. Experimental Gerontology. 2002;37:477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 35.McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Research Reviews. 2002;1:79–93. doi: 10.1016/s0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- 36.Adhihetty PJ, Irrcher I, Joseph A, Ljubiicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Experimental Physiology. 2003;88:99–107. doi: 10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- 37.Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochem Biophys Acta. 1994;1226:73–82. doi: 10.1016/0925-4439(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 38.Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- 39.Katz A, Nambi SS, Mather K, et al. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 40.Bruce CR, Anderson MJ, Carey AL, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. Journal of Clinical Endocrinology & Metabolism. 2003;88:5444–5451. doi: 10.1210/jc.2003-030791. [DOI] [PubMed] [Google Scholar]
- 41.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 42.Rimbert V, Boirie Y, Bedu M, Hocquette JF, Ritz P, Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB Journal. 2004;18:737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- 43.Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 44.Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288:C1074–1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]


