When Shakespeare wrote (46), “What's in a name? that which we call a rose / By any other name would smell as sweet,” he implied that phenotypes (scent in this case) take precedence over nomenclature. In popular usage, they usually do. Cartoonists classify politicians by their ears or noses. Scientists use physical characteristics to delimit everything from species (e.g., cranium size in the genus Homo) to kingdoms. Throughout much of taxonomic history, macroscopic characters have been preferred for obvious reasons.
EPOCHS IN TAXONOMY
As individual bacteria are too small to see, their classification presents special difficulties. van Leeuwenhoek's invention of the microscope not only rendered bacteria visible (9) but also permitted sorting them into morphological groups (cocci, spirals, and short and elongated rods [8]). In 1884 Christian Gram devised a procedure that separated bacteria into two major staining-reaction groups (47). A second era began when biochemical and physiological characters were used to identify and classify cultures (36). A third revolution followed Sanger, Gilbert, and Maxam's development of methods for sequencing DNA in the 1970s (2, 23). Sequence variation in genes that encode essential functions is obviously restricted to those base changes that do not affect viability. It is assumed that any changes that have occurred must have been acquired slowly and possibly also at a constant rate. Obviously, transcription and translation are central to all organisms, and for this reason ribosomal genes have found particular favor.
In other words, technological advances have driven each of the three (the morphological, the physiological, and the sequence) epochs of bacterial taxonomy. As with all new methods, they have to be finely tuned before they are of widespread utility, and as the paper by van Berkum et al. in this issue (57) shows, attempts to use sequence data to classify bacteria need reexamination.
Symbiotic, nitrogen-fixing bacteria interact with legumes in a readily identifiable manner (producing root nodules). Partly for this reason, they have been classified and studied since the dawn of bacteriology. Bacillus radiocola was probably the first name used, but when Nobbe et al. (32, 33) found that bacteria isolated from Pisum sativum nodules were unable to nodulate plants belonging to the legume tribes Genisteae and Hedysareae, a simple solution presented itself—to name the bacterium after the host plant (19). Later, many taxonomic proposals were made (for examples, see reference 16), but all strongly emphasized the host from which the Rhizobium was isolated (28, 51, 60).
There are many problems with this approach, including the fact that about 18,000 species of legumes as well as countless rhizobia exist. Also, the “host range” of both bacteria and plants varies from pairs that are more or less faithful to one another to combinations in which almost all traces of specificity have vanished (4, 38). As examples, a number of genera within the Phaseoleae (e.g., Phaseolus and Vigna) form nodules with about half of all rhizobia presented to them (27, 31) and some individual rhizobia (e.g., the broad host range Rhizobium species NGR234) are able to nodulate about 50% of all legumes (41). A group such as the “cowpea” miscellany (by definition, members of this group nodulate cowpea [Vigna unguiculata] in addition to the host from which they were isolated) eventually contained rhizobia isolated from the majority of all nodulated legumes (34).
LA MODE—THE 16S rRNA GENE
As similar problems existed with other groups (e.g., Pseudomonas [37]), taxonomists desperately sought new methods to classify bacteria. Characters such as DNA base ratios, amino acid sequences of proteins, DNA-DNA as well as DNA-RNA hybridizations, the constituents of ribosomes and of cell walls, etc., have all been used, often with surprising consequences. Reviewing this work in 1981, Trüper and Krämer (53) asked, “Which systematic basis will prevail; morphology, physiology or chemical composition of cellular components?” and then replied, “There is no answer yet to the question and there may never be a final answer.” Nevertheless, sequencing conserved genes (or parts of genes) is a simple way to provide insights that elude morphological and physiological methods. In themselves, improvements in sequencing technologies would have accelerated the use of sequence data in bacterial taxonomy, but a further development, that of the PCR, greatly simplified the task. Carefully designed oligonucleotide primers allowed amplification and sequencing of only the variable portion of a target gene that could be as short as 200 bp. A single sequencing gel could thus provide taxonomic information on many accessions. Furthermore, these same techniques could be applied to nonpurified DNA or even to “environmental samples.” An explosion of papers purporting new taxonomic relationships resulted. Some of them were greeted with enthusiasm, while others seeded confusion.
Using sequence variation of the 16S rRNA gene (or any other gene for that matter) for taxonomic purposes presupposes that evolution of the genome progresses at a constant rate and that genes are inherited in a strictly hierarchical manner—in other words, that genes are passed from generation to generation and are not shared between existing cells via horizontal or lateral transfer. Suspicions that this might not always be the case arose from the findings that many taxa, including Clostridium (42), Escherichia coli (seven alleles) (7), Haloarcula (5% difference between the two expressed copies [1]), and Rhodobacter (12), contain multiple and often-divergent 16S rRNA genes. The most damning example is that of Thermobispora bispora, however, which contains two similar copies of the 16S rRNA gene (as well as three copies of the 23S rRNA gene) that differ from each other by 6.4% at the nucleotide level (59). As these copies of the 16S rRNA gene are on the same chromosome within the same cell, their sequence divergence suggests that the rather arbitrary 5% mismatch that had previously been used to place bacteria into separate genera is untenable.
INCONSISTENCIES IN 16S rRNA, ITS, AND 23S rRNA SEQUENCES
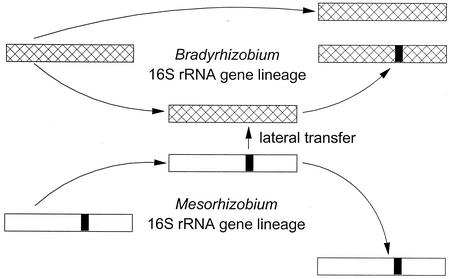
Reexamination of this problem by van Berkum et al. (57) as it applies to the Rhizobiaceae is timely not only because of these problems but also because Young et al. (62) claim that the close relatedness of 16S rRNA sequences of Agrobacterium and Rhizobium species (<7% mismatch) warrants regrouping the agrobacteria and rhizobia into a single genus, Rhizobium. What van Berkum et al. did was to sequence the 16S rRNA and the 23S rRNA genes as well as the internally transcribed space (ITS) region that is located between the conserved portions at the 3′ end of the 16S rRNA gene and the 5′ end of the 23S rRNA gene of a number of α-Proteobacteria (Agrobacterium, Rhizobium, and related genera). Standard computational analyses were then performed on these sequence data to construct phylogenetic relationships among the bacteria. Their results show that the ITS region and the 23S rRNA gene provide phylogenetic signals which are different from those derived from the 16S rRNA gene. In other words, the three sets of data produced three morphologically distinct phylogenetic trees that are impossible to combine into a single tree. In part, this is due to multiple copies of the 16S rRNA gene referred to above (which copy is representative of the species?), but the major contribution of van Berkum et al. concerns the discovery that allelic variation within the rrn locus is due to gene conversion. Their data show that a small portion of the 16S rRNA gene of Bradyrhizobium elkanii originated from Mesorhizobium by lateral transfer (Fig. 1). If this is true, it negates the principle that rRNA genes are inherited only by vertical descent (see above). And if mother-to-daughter transfer is not the only mechanism by which rRNA genes are inherited, further use of 16S rRNA sequence data to construct phylogenetic trees is no longer justified.
FIG. 1.
Model showing how recombination between short segments of the 16S rRNA genes of B. elkani and species of Mesorhizobium may have occurred, resulting in the lateral transfer of the 16S rRNA gene from Mesorhizobium to Bradyrhizobium. See the text for further details.
GENE CONVERSION
Lateral transfer of genes is known to produce extremely dynamic genomes in which substantial amounts of DNA are introduced into and deleted from bacterial chromosomes (35). To test whether gene conversion is at least partly responsible for the discordant phylogenies within the Rhizobiaceae, van Berkum et al. searched among specific alleles of the 16S rRNA genes that may have a history of recombination. Potential recombination events between short segments of the 16S rRNA genes of B. elkani and species of Mesorhizobium, as well as between Sinorhizobium and Mesorhizobium, were identified (see Fig. 5 in reference 57). This suggests that divergent genera of the α-Proteobacteria are not as genetically isolated as previously claimed (17).
For gene conversion to occur, bacteria must exchange genetic information among themselves. Do they? Laboratory experiments have clearly shown that Agrobacterium tumefaciens carrying symbiotic (Sym) plasmids of various Rhizobium species produce atypical, Fix− nodules (3, 6, 20, 21, 24, 52, 61), although A. tumefaciens containing a Rhizobium etli plasmid forms nitrogen-fixing nodules (29). Ti plasmids of A. tumefaciens are self-conjugal elements (13). Nevertheless, despite proper virulence gene induction and T-strand formation, transconjugants of Rhizobium meliloti harboring Ti plasmids of A. tumefaciens do not produce tumors on plants (58), suggesting that genetic barriers between the two organisms exist. Here the point is not that Agrobacterium harboring Rhizobium plasmids produces effective, nitrogen-fixing nodules on legumes (or that Rhizobium transconjugants containing Ti plasmids provoke crown galls) but that the plasmids are maintained in the heterologous backgrounds, and this is plainly the case.
Thus, the next question is: does horizontal transfer of genetic information occur under natural conditions, e.g., in the rhizosphere? Two different Sym plasmids of Rhizobium leguminosarum readily complemented a nonattaching, nonnodulating mutant of R. meliloti in the rhizosphere of Medicago sativa (5). Although certain plasmid-chromosome combinations are favored, natural populations of R. leguminosarum also display extensive transfer of symbiotic plasmids in the field (18, 26, 43, 45). Moreover, structural rearrangements among the plasmids of the transconjugants also occur (18), using well-documented mechanisms (15, 30, 44). Undoubtedly, the most striking evidence of horizontal transfer concerns the “symbiosis islands” of Mesorhizobium loti. Genetically diverse “mesorhizobia” were isolated from nodules of Lotus corniculatus growing in fields that were devoid of indigenous Lotus rhizobia, but which had been inoculated with a single M. loti isolate (48). All contained a 502-kb chromosomally integrated element that transfers to nonsymbiotic mesorhizobia, converting them to Lotus symbionts. This symbiotic island integrates into a phenylalanine tRNA gene on the chromosome of the host, in a process mediated by a P4-type integrase encoded at one end of the element (48-50).
NAMES OF THE ROSES
There is little doubt that soil bacteria are not unchangeable, static organisms. On the contrary, plasmids and well-defined parts of chromosomes are freely exchanged among bacteria, especially when they congregate at the root surface (the rhizoplane) or within the nodule (40). Furthermore, a small (53-kb) plasmid of Bacillus megaterium harbors a functional rRNA operon that is probably transferable to other bacteria (25). Since bacterial genomes are much more fluid than previously thought, there is little reason to doubt that acquisition of foreign DNA, followed by recombination into the parental genome, is an important driving force in evolution. That essential genes are targets for conversion may come as a surprise, but as Flores et al. (15) have shown, repeated sequences are “hot spots” for genomic rearrangements. As complete DNA sequences of other Rhizobiaceae become available (at the time of writing, only those of A. tumefaciens, Bradyrhizobium japonicum, M. loti, and R. meliloti have been published), more concatameric 16S rRNA genes will undoubtedly be found. In their paper, van Berkum et al. (57) suggest that rather than being the dominant character used in bacterial taxonomy, the DNA sequence of the 16S rRNA gene should be only one of many used. If this principle is to be applied, it means, however, that some of the recent name changes based on analysis of the 16S rRNA gene need to be rethought (Table 1). Several groups have made cogent arguments against the adoption of the new names (14, 52-54). The report by Farrand et al. (14) also contains a list of over 100 bacteriologists who are opposed to the proposal of Young et al. (62).
TABLE 1.
Proposed changes in the nomenclature of some genera and species of the Rhizobiaceae based primarily on the DNA sequence of the 16S rRNA genea
| Old name | Proposed or new name | Special features | Reference(s) | Suggested name |
|---|---|---|---|---|
| A. tumefaciens (bv. 1) (includes Agrobacterium rubi) | Rhizobium radiobacter | Provokes galls; circular and linear chromosomes; no RIMEsb | 14 vs 62 | Agrobacterium tumefaciens (tumor forming, regard- less of biovar) |
| Agrobacterium rhizogenes (bv. 2) | Rhizobium rhizogenes | Provokes hairy roots | 14 vs 62 | A. rhizogenes (root forming, regardless of biovar) |
| A. rubi | Rhizobium rubi | Provokes galls | 14 vs 62 | A. rubi |
| Agrobacterium vitis | Rhizobium vitis | Provokes galls | 14 vs 62 | A. vitis (bv. 3) |
| Allorhizobium undicola | Rhizobium undicola | Nodulates Neptunia natans | 11 and 62 vs 14 | R. undicola |
| Mesorhizobium loti MAFF303099 | Mesorhizobium huakuii bv. loti | Nodulates Lotus corniculatus; completely sequenced | 54 | M. huakuii |
| Rhizobium | Sinorhizobium | Nodulates many legumes; two circular chromosomes; many RIMEs | 10 vs 14 and 55,56,57 | Rhizobium (or Sinorhizobiumc); R. fredii, R. meliloti, R. saheli, R. terangae, etc. |
As the scientific basis for these name changes has been questioned by the findings of others (listed under references), I propose that the former names be used until a detailed revision of the family is made.
RIMEs, Rhizobium-specific intergenic mosaic elements.
Although a consensus is now forming that changing the name from Rhizobium to Sinorhizobium is not warranted (14, 55-57), recently many authors adopted the convention of referring to some of these bacteria as Sinorhizobium. It goes against the spirit of this commentary to dictate that Sinorhizobium should be abandoned at this time. Furthermore, Euzéby (http://www.bacterio.cict.fr) said “…it is possible for two or more validly published names to remain in use.”
There are really only two reasons for giving names to living objects—to pinpoint them so that others will understand which one is being talked about and, if possible, to group them so that their interrelationships are obvious. Essentially, these are the differences between taxonomy (which could be achieved by a sort of “bacterial bar code”) and phylogeny, which is the evolutionary history of a species or other taxonomic group. Superficially, many flowers look like roses but their scent sets them apart. So too with the Rhizobiaceae—Agrobacterium makes crown galls, Rhizobium makes nodules. One is a pathogen, the other is a symbiont. Whether or not these traits are reflected in the 16S rRNA sequence is of lesser importance in giving names, since we have an obligation to ensure (i) that the name reflects an easily discernible reality (e.g., a rose, a gall, or a nodule), (ii) that the name is not a source of error, (iii) that the name is not equivocal, (iv) that the name is maintained for as long as possible, and (v) that the name is commonly accepted.
van Berkum et al. (57) have done the scientific community a large service by pointing out that names based solely on 16S rRNA sequence data satisfy few of these criteria. Or, as Postgate (39) wrote, “…new rRNA phylogeny is the phylogeny of rRNA genes, not of their hosts… .” A moratorium or at least a cooling-down period on renaming the Rhizobiaceae (and probably other groupings) is thus called for. It would be sensible to wait until further data are available on a variety of conserved genes (23S rRNA, the ITSs, glnA, nodA, recA, etc.). Some of this will be provided by current whole-genome sequencing projects, but more could be gathered by using current techniques (54, 57). When data are available, and after a suitable period of reflection, perhaps it would be appropriate if the editor of the Journal of Bacteriology or the editor of the International Journal of Systematic and Evolutionary Bacteriology commissioned an “outsider” to revise the genera Agrobacterium and Rhizobium, etc., which would be published in their respective journals.
One final point concerns precedent. Many think that if there are compelling morphological and behavioral reasons for reclassifying competitors as Rattus erectus, rules of precedent require that if this is published, the name R. erectus would have to be used in place of Homo sapiens sapiens in the scientific literature. This is not the case. Extracts from J. P. Euzéby's List of Bacterial Names with Standing in Nomenclature (http://www.bacterio.cict.fr) (updated 28 January 2003) include the following:
(i) “There is no official classification of bacteria, but the names given to bacteria are regulated.”
(ii) “… the name of a taxon is validly published, and therefore has standing in nomenclature, if one of the following criteria is met: 1) the name is cited in the Approved Lists of Bacterial Names. 2) The name is published in papers in the International Journal of Systematic and Evolutionary Microbiology (and its predecessor). 3) The name is validated by announcement in a Validation List.”
(iii) But in a nota bene he adds, “1) The names in this list are ‘valid’ only in the sense of being validly published as a result of conformity with the Rules of Nomenclature. The names which are to be used are those which are correct in the opinion of the bacteriologist (especially a combinatio nova or a nomen novum), and a particular name does not have to be adopted… ..” This was confirmed by the International Committee on Systematics of Prokaryotes (22), who said, “Consequently, the committee suggest that it is up to the individual experts and/or authors to choose… which name they want to use.”
The “take-home message” is thus clear. Use the names that you think best describe the organism in light of the five taxonomic rules mentioned above. In time, rhizobial taxonomy will stabilize and form a consensus that we can all live with, and van Berkum et al. will be thanked for helping with that.
Acknowledgments
I thank W. J. Deakin, S. K. Farrand, P. J. J. Hooykaas, P. Mavingui, R. Palacios, X. Perret, M. J. Sadowsky, R. Spichiger, G. Stacey, and G. C. Walker for their many helpful comments on the manuscript, as well as D. Gerber for general support.
Research in LBMPS is financed by the Fonds National de la Recherche Scientifique (Project 31-63893.00) and the Université de Genève.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Amann, G., K. O. Stetter, E. Llobet-Brossa, R. Amann, and J. Anton. 2000. Direct proof for the presence and expression of two 5% different 16S rRNA genes in individual cells of Haloarcula marismortui. Extremophiles 4:373-376. [DOI] [PubMed] [Google Scholar]
- 2.Brenner, S., and J. H. Miller. 2002. Encyclopedia of genetics. Academic Press, London, United Kingdom.
- 3.Broughton, W. J., N. Heycke, H. Meyer z. A., and C. E. Pankhurst. 1984. Plasmid-linked nif and “nod” genes in fast growing rhizobia that nodulate Glycine max, Psophocarpus tetragonolobus and Vigna unguiculata. Proc. Natl. Acad. Sci. USA 81:3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broughton, W. J., S. Jabbouri, and X. Perret. 2000. Keys to symbiotic harmony. J. Bacteriol. 182:5641-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broughton, W. J., U. Samrey, and J. Stanley. 1987. Ecological genetics of Rhizobium meliloti: symbiotic plasmid transfer in the Medicago sativa rhizosphere. FEMS Microbiol. Lett. 40:251-255. [Google Scholar]
- 6.Broughton, W. J., C. H. Wong, A. Lewin, U. Samrey, H. Myint, H. Meyer z. A., D. N. Dowling, and R. Simon. 1986. Identification of Rhizobium plasmid sequences involved in recognition of Psophocarpus, Vigna and other legumes. J. Cell Biol. 102:1173-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbon, C., J. Philips, Z. Y. Fu, C. Squires, and C. L. Squires. 1976. The complete nucleotide sequence of ribosomal 16S rRNA from Escherichia coli. EMBO J. 11:4175-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn, F. 1872. Untersuchungen über Bacterien. II. Beiträge zur Biologie der Pflanzen 1:127-224.
- 9.de Kruif, P. 1926. Microbe hunters. Harcourt, Brace & Co., New York, N.Y.
- 10.de Lajudie, P., A. Willems, B. Pot, D. Dewettinck, G. Maestrojuan, M. Neyra, M. D. Collins, B. Dreyfus, K. Kersters, and M. Gillis. 1994. Polyphasic taxonomy of rhizobia: emendation of the genus Sinorhizobium and description of Sinorhizobium meliloti comb. nov. Sinorhizobium saheli sp. nov. and Sinorhizobium teranga sp. nov. Int. J. Syst. Bacteriol. 44:715-733. [Google Scholar]
- 11.de Lajudie, P., E. Laurent-Fulele, A. Willems, U. Torck, R. Coopman, M. D. Collins, K. Kersters, B. Dreyfus, and M. Gillis. 1998. Allorhizobium undicola gen. nov., sp. nov., nitrogen-fixing bacteria that efficiently nodulate Neptunia natans in Senegal. Int. J. Syst. Bacteriol. 48:1277-1290. [DOI] [PubMed] [Google Scholar]
- 12.Dreyden, S. C., and S. Kaplan. 1990. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 18:7267-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrand, S. K. 1998. Conjugal plasmids and their transfer, p. 199-233. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae. Molecular biology of model plant-associated bacteria. Kluwer Academic Publ., Dordrecht, The Netherlands.
- 14.Farrand, S. K., P. B. van Berkum, and P. Oger. 13 December 2002. Agrobacterium is a definable genus of the family Rhizobiaceae. Int. J. Syst. Evol. Microbiol. 10.1099/ijs0.02445-0. [DOI] [PubMed]
- 15.Flores, M., P. Mavingui, X. Perret, W. J. Broughton, D. Romero, G. Hernandez, G. Davila, and R. Palacios. 2000. Prediction, identification, and artificial selection of DNA rearrangements in Rhizobium: toward a natural genomic design. Proc. Natl. Acad. Sci. USA 97:9138-9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fred, E. B., I. L. Baldwin, and E. McCoy. 1932. Root nodule bacteria and leguminous plants. University of Wisconsin Press, Madison, Wis.
- 17.Gaunt, M. W., S. L. Turner, L. Rigottier-Gois, S. A. Lloyd-Macgilp, and J. P. W. Young. 2001. Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int. J. Syst. Bacteriol. 51:2037-2048. [DOI] [PubMed] [Google Scholar]
- 18.Geniaux, E., and N. Amarger. 1993. Diversity and stability of plasmid transfer in isolates from a single field population of Rhizobium leguminosarum bv. viciae. FEMS Microbiol. Ecol. 102:251-260. [Google Scholar]
- 19.Hiltner, L., and K. Störmer. 1903. Neue Untersuchungen über die Wurzelknöllchen der Leguminosen und deren Erreger. Arbeiten aus der Biologischen Abteilung für Land- und Forstwirthschaft, Kaiserlichen Gesundheitsamte, Berlin 3:151-307. [Google Scholar]
- 20.Hooykaas, P. J. J., A. A. N. van Brussel, H. den Dulk-Ras, G. M. S. van Slogteren, and R. A. Schilperoort. 1981. Sym plasmid of Rhizobium trifolii expressed in different rhizobial species and in Agrobacterium tumefaciens. Nature 291:351-353. [Google Scholar]
- 21.Hooykaas, P. J. J., G. M. Snijdewint, and R. A. Schilperoort. 1982. Identification of the Sym plasmid of Rhizobium leguminosarum strain 1001 and its transfer to and expression in other rhizobia and Agrobacterium tumefaciens. Plasmid 8:73-82. [DOI] [PubMed] [Google Scholar]
- 22.International Committee on Systematics of Prokaryotes. 2002. Subcommittee on the taxonomy of Agrobacterium and Rhizobium. Int. J. Syst. Evol. Microbiol. 52:2337. [Google Scholar]
- 23.Janda, J. M., and S. L. Abbott. 2002. Bacterial identification for publication: when is enough enough? J. Clin. Microbiol. 40:1887-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondorosi, A., C. E. Pankhurst, W. J. Broughton, and Z. Banfalvi. 1982. Mobilization of a Rhizobium meliloti megaplasmid carrying nodulation and nitrogen fixation genes into other rhizobia and Agrobacterium. Mol. Gen. Genet. 188:433-439. [Google Scholar]
- 25.Kunnimalaiyaan, M., D. M. Stevenson, Y.-S. Zhou, and P. S. Vary. 2001. Analysis of the replicon region and identification of an rRNA operon on pBM400 of Bacillus megaterium QM B1551. Mol. Microbiol. 39:1010-1021. [DOI] [PubMed] [Google Scholar]
- 26.Laguerre, G., E. Geniaux, S. I. Mazurier, R. R. Casartelli, and N. Amarger. 1993. Conformity and diversity among field isolates of Rhizobium leguminosarum bv viciae, bv trifolii, and bv phaseoli revealed by DNA hybridisation using chromosome and plasmid probes. Can. J. Microbiol. 39:412-419. [Google Scholar]
- 27.Lewin, A., C. Rosenberg, H. Meyer z. A., C. H. Wong, L. Nelson, J.-F. Manen, J. Stanley, D. N. Dowling, J. Dénarié, and W. J. Broughton. 1987. Multiple host-specificity loci of the broad host range Rhizobium sp. NGR 234 selected using the widely compatible legume Vigna unguiculata. Plant Mol. Biol. 8:447-459. [DOI] [PubMed] [Google Scholar]
- 28.Lim, G., and J. C. Burton. 1982. Nodulation status of the Leguminosae, p. 1-34. In W. J. Broughton (ed.), Nitrogen fixation. II. Rhizobium. Oxford University Press, Oxford, United Kingdom.
- 29.Martinez, E., R. Palacios, and F. Sanchez. 1987. Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J. Bacteriol. 169:2828-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mavingui, P., M. Flores, X.-W. Guo, G. Dávila, X. Perret, W. J. Broughton, and R. Pacacios. 2002. Dynamics of genome architecture in Rhizobium sp. strain NGR234. J. Bacteriol. 184:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michiels, J., B. Dombrecht, N. Vermeiren, C.-W. Xi., E. Luyten, and J. Vanderleyden. 1998. Phaseolus vulgaris is a non-selective host for nodulation. FEMS Microbiol. Ecol. 26:193-205. [Google Scholar]
- 32.Nobbe, F., L. Hiltner, and E. Schmid. 1895. Versuche über die Biologie der Knöllchenbakterien der Leguminosen, insbesondere über die Frage der Arteinheit derselben. Landwirtschaftlichen Versuchstationen Dresden 45:1-27.
- 33.Nobbe, F., E. Schmid, L. Hiltner, and E. Hotter. 1891. Versuche über die Stickstoff-Assimilation der Leguminosen. Landwirtschaftlichen Versuchstationen Dresden 39:327-359.
- 34.Norris, D. O. 1956. Legumes and the Rhizobium symbiosis. Empire J. Exptl. Agric. 24:247-270. [Google Scholar]
- 35.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 36.Orla-Jensen, S. 1909. Die Hauptlinien des natürlichen Baktieren Systems. Centralblatt für Bakteriologie, Abt. II 22:305-346. [Google Scholar]
- 37.Palleroni, N. J. 2003. Prokaryote taxonomy of the 20th century and the impact of studies on the genus Pseudomonas: a personal view. Microbiology 149:1-7. [DOI] [PubMed] [Google Scholar]
- 38.Perret, X., C. Staehelin, and W. J. Broughton. 2000. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 64:180-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postgate, J. 1995. Breathless niches. Nature 377:26. [Google Scholar]
- 40.Pretorius-Güth, I. M., A. Pühler, and R. Simon. 1990. Conjugal transfer of mega-plasmid 2 between Rhizobium meliloti strains in alfalfa nodules. Appl. Environ. Microbiol. 56:2354-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pueppke, S. G., and W. J. Broughton. 1999. Rhizobium sp. NGR234 and R. fredii USDA257 share exceptionally broad, nested host-ranges. Mol. Plant-Microbe Interact. 12:293-318. [DOI] [PubMed] [Google Scholar]
- 42.Rainey, F. A., N. L. Ward-Rainey, P. H. Janssen, H. Hippe, and E. Stackebrandt. 1996. Clostridium paradoxum DSM 7308T contains multiple 16S rRNA genes with heterogeneous intervening sequences. Microbiology 142:2087-2095. [DOI] [PubMed] [Google Scholar]
- 43.Rigottier-Gois, L., S. L. Turner, J. P. W. Young, and N. Amarger. 1998. Distribution of repC plasmid-replication sequences among plasmids and isolates of Rhizobium leguminosarum bv. viciae from field populations. Microbiology 144:771-780. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez, C., and D. Romero. 1998. Multiple recombination events maintain sequence identity among members of the nitrogenase multigene family in Rhizobium etli. Genetics 149:785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schofield, P. R., A. H. Gibson, W. F. Dudman, and J. M. Watson. 1987. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmids in a soil population. Appl. Environ. Microbiol. 53:2942-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shakespeare, W. 1993, posting date. The complete works of William Shakespeare [Online.] http://the-tech.mit.edu/Shakespeare/.
- 47.Starr, M. P., and J. M. Schmidt. 1981. Prokaryote diversity, p. 3-42. In M. P. Starr, H. Stolp, H. G. Trüper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes. A handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, Berlin, Germany.
- 48.Sullivan, J. T., H. N. Patrick, W. L. Lowther, D. B. Scott, and C. W. Ronson. 1995. Nodulating strains of Rhizobium loti arise through chromosomal symbiotic gene transfer in the environment. Proc. Natl. Acad. Sci. USA 92:8985-8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan, J. T., and C. W. Ronson. 1998. Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc. Natl. Acad. Sci. USA 95:5145-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan, J. T., J. R. Trzebiatowski, R. W. Cruickshank, J. Gouzy, S. D. Brown, R. M. Elliot, D. J. Fleetwood, N. G. McCallum, U. Rossbach, G. S. Stuart, J. E. Weaver, R. J. Webby, F. J. de Bruijn, and C. W. Ronson. 2002. Comparative sequence analysis of the symbiosis island of Mesorhizobium loti strain R7A. J. Bacteriol. 184:3086-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trinick, M. J. 1982. Biology, p. 1-34. In W. J. Broughton (ed.,) Nitrogen fixation. II. Rhizobium. Oxford University Press, Oxford, United Kingdom.
- 52.Truchet, G., G. Rosenberg, J. Vasse, J.-S. Julliot, S. Camut, and J. Dénarié. 1984. Transfer of Rhizobium meliloti pSym genes into Agrobacterium tumefaciens: host-specific nodulation by atypical infection. J. Bacteriol. 157:134-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trüper, H. G., and J. Krämer. 1981. Principles of characterization and identification of prokaryotes, p. 176-193. In M. P. Starr, H. Stolp, H. G. Trüper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes. A handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, Berlin, Germany.
- 54.Turner, S. L., X.-X. Zhang, F.-D. Li, and J. P. W. Young. 2002. What does a bacterial genome sequence represent? Mis-assignment of MAF303099 to the genospecies Mesorhizobium loti. Microbiology 148:3330-3331. [DOI] [PubMed] [Google Scholar]
- 55.van Berkum, P., and B. D. Eardly. 2003. Impact of genomics on the reconstruction of evolutionary relationships of nitrogen-fixing bacteria and implications for taxonomy, p. 1-21. In R. Palacios and W. E. Newton (ed.), Nitrogen fixation: 1888-2001. VI. Genomes and genomics of nitrogen-fixing organisms. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 56.van Berkum, P., J. J. Fuhrmann, and B. D. Eardly. 2000. Phylogeny of rhizobia, p. 165-169. In F. O. Pedrosa, M. Hungria, G. Yates, and W. E. Newton (ed.), Nitrogen fixation: from molecules to crop productivity. Kluwer Academic Publ., Dordrecht, The Netherlands.
- 57.van Berkum, P., Z. Terefework, L. Paulin, S. Suomalainen, K. Lindström, and B. D. Eardly. 2003. Discordant phylogenies with the rrn loci of rhizobia. J. Bacteriol. 185:2988-2998. [DOI] [PMC free article] [PubMed]
- 58.van Veen, R. J. M., H. den Dulk-Ras, R. A. Schilperoort, and P. J. J. Hooykaas. 1989. Ti plasmid containing Rhizobium meliloti are non-tumorigenic on plants, despite proper virulence gene induction and T-strand formation. Arch. Microbiol. 153:85-98. [Google Scholar]
- 59.Wang, Y., Z. Zhang, and N. Ramanan. 1997. The actinomycete Thermobispora bispora contains two distinct types of transcriptionally active 16S rRNA genes. J. Bacteriol. 179:3270-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson, J. K. 1939. Leguminous plants and their associated organisms. Cornell Univ. Agric. Exp. Station Memoir 221. Cornell University Press, Ithaca, N.Y.
- 61.Wong, C. H., C. E. Pankhurst, A. Kondorosi, and W. J. Broughton. 1983. Morphology of root-nodules and nodule-like structures formed by Rhizobium and Agrobacterium strains containing a R. meliloti megaplasmid. J. Cell Biol. 97:787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young, J. M., L. D. Kuykendall, E. Martinez-Romero, A. Kerr, and H. Sawada. 2001. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 51:89-103. [DOI] [PubMed] [Google Scholar]