Abstract
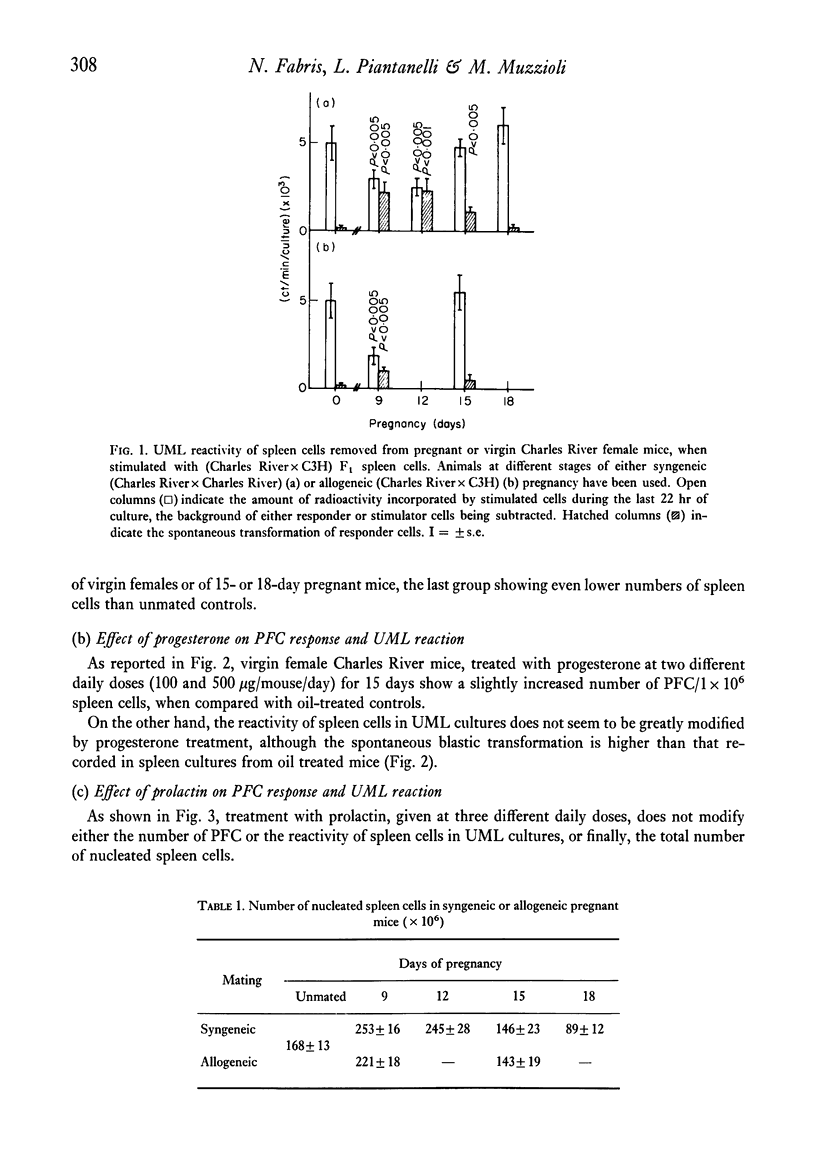
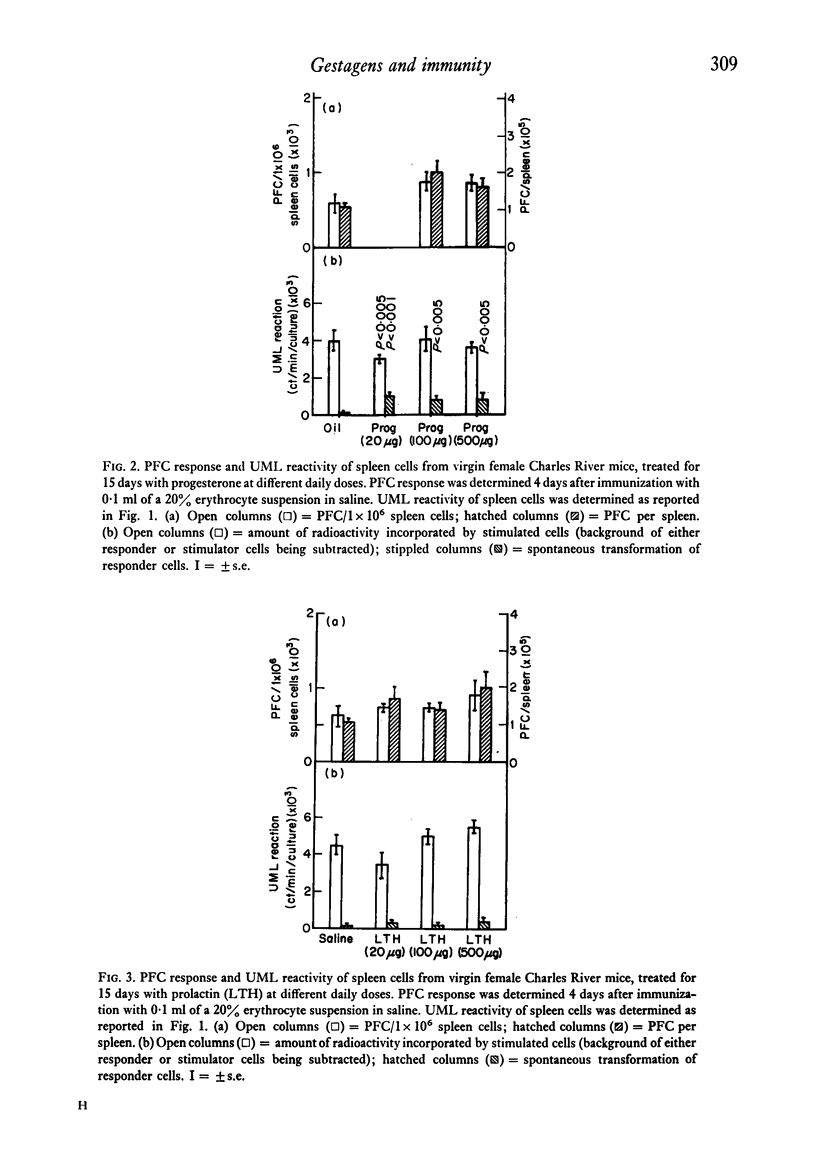
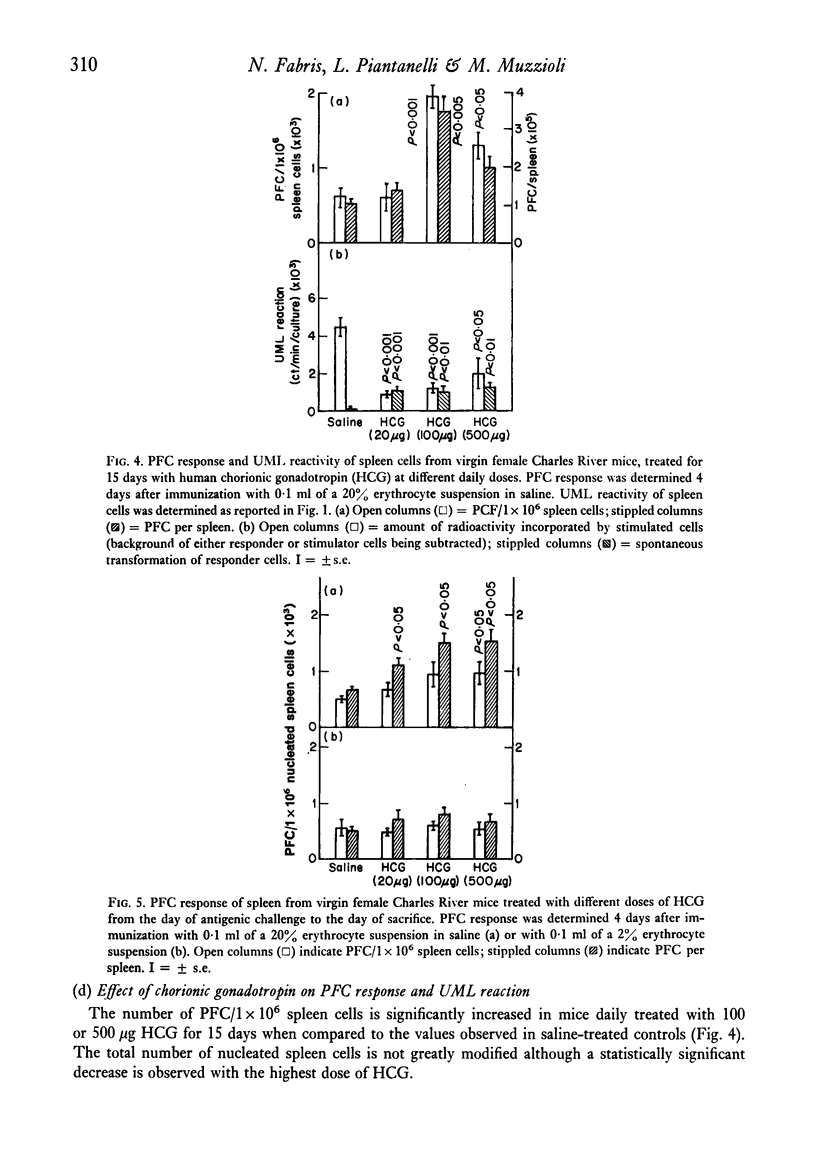
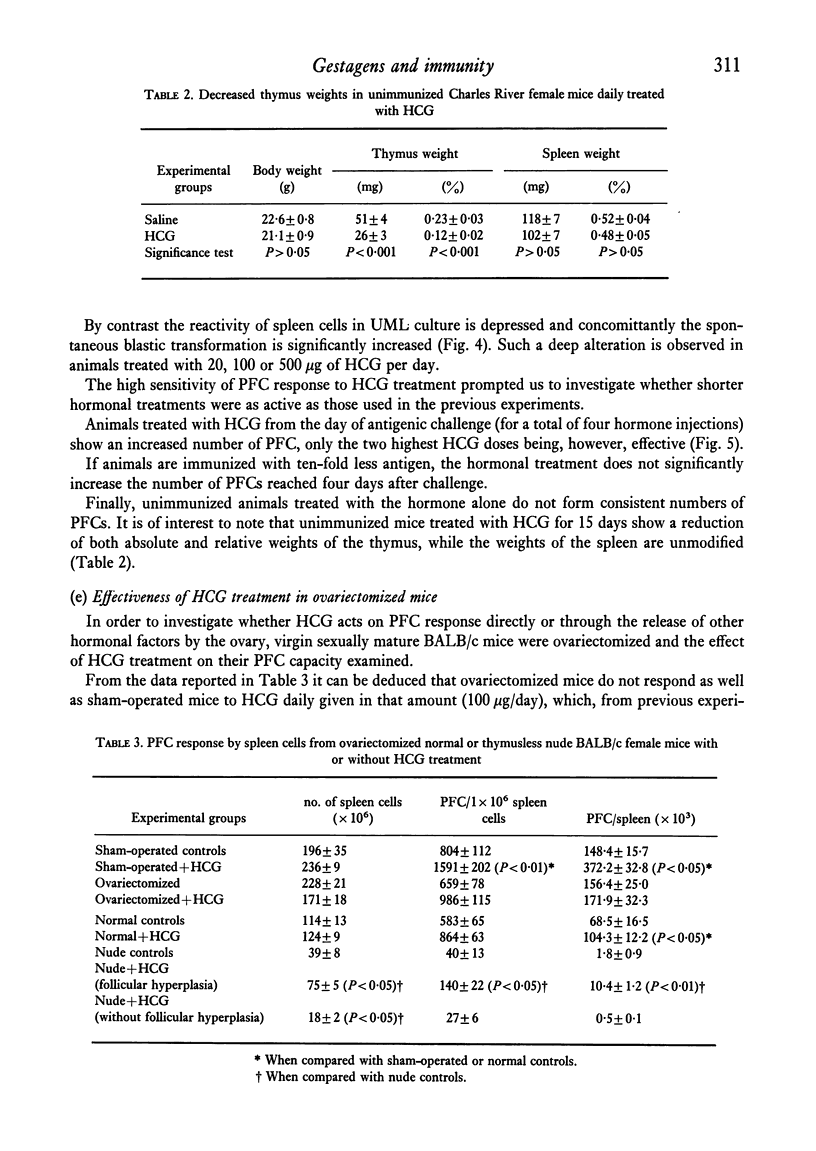
The reactivity of spleen lymphocytes in a mixed lymphocyte culture and the in vivo PFC response to sheep erythrocytes have been evaluated in pregnant female mice and data compared with those observed in virgin sexually mature female mice daily treated either with progesterone or human chorionic gonadotropin (HCG) or human prolactin. The mixed lymphocyte reactivity is depressed at mid-pregnancy, whereas PFC response is increased. Comparable immunological modifications have been found in mice treated with HCG, but not in animals treated with progesterone or prolactin. The similarity between HCG treatment and pregnancy suggests that the rate of gonadotropin release may be one of the earliest events responsible for the immunological disturbances present during pregnancy, although its action on the lymphoid system seems to require the presence of the ovary. From these data and from the observation that HCG increases the PFC response also in thymusless nude female mice, it can be deduced that it acts on both T and B cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. W., MacCuish A. C., Loudon N. B., Jordan J., Irvine W. J. Phytohaemagglutinin-induced lymphocyte transformation and circulating autoantibodies in women taking oral contraceptives. Lancet. 1974 May 11;1(7863):898–900. doi: 10.1016/s0140-6736(74)90348-1. [DOI] [PubMed] [Google Scholar]
- Bentley H. P., Hughs E. R., Peterson R. D. Effect of hypophysectomy on a virus-induced T-cell leukemia. Nature. 1974 Dec 20;252(5485):747–748. doi: 10.1038/252747a0. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., Sorkin E. Thymus involvement in female sexual maturation. Nature. 1974 May 24;249(455):356–358. doi: 10.1038/249356a0. [DOI] [PubMed] [Google Scholar]
- Carr M. C., Stites D. P., Fudenberg H. H. Cellular immune aspects of the human fetal maternal relationship. II. In vitro response of gravida lymphocytes to phytohemagglutinin. Cell Immunol. 1973 Sep;8(3):448–454. doi: 10.1016/0008-8749(73)90136-6. [DOI] [PubMed] [Google Scholar]
- Contractor S. F., Davies H. Effect of human chorionic somatomammotrophin and human chorionic gonadotrophin on phytohaemagglutinin-induced lymphocyte transformation. Nat New Biol. 1973 Jun 27;243(130):284–286. doi: 10.1038/newbio243284a0. [DOI] [PubMed] [Google Scholar]
- Davies I. J., Ryan K. J. Comparative endocrinology of gestation. Vitam Horm. 1972;30:223–279. doi: 10.1016/s0083-6729(08)60797-9. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris N. Immunodepression in thyroid-deprived animals. Clin Exp Immunol. 1973 Dec;15(4):601–611. [PMC free article] [PubMed] [Google Scholar]
- Fabris N. Immunological reactivity during pregnancy in the mouse. Experientia. 1973 May 15;29(5):610–612. doi: 10.1007/BF01926697. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I., Brawn J. Abrogation of cellular immunity to antigenically foreign mouse embryonic cells by a serum factor. Nature. 1969 Nov 29;224(5222):914–915. doi: 10.1038/224914a0. [DOI] [PubMed] [Google Scholar]
- Hill C. A., Finn R., Denye V. Depression of cellular immunity in pregnancy due to a serum factor. Br Med J. 1973 Sep 8;3(5879):513–514. doi: 10.1136/bmj.3.5879.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakura S. Is cortisol responsible for inhibition of MLC reactions by pregnancy plasma? Nature. 1973 Dec 21;246(5434):496–497. doi: 10.1038/246496a0. [DOI] [PubMed] [Google Scholar]
- Morton H., Hegh V., Clunie G. J. Immunosuppression detected in pregnant mice by rosette inhibition test. Nature. 1974 May 31;249(456):459–460. doi: 10.1038/249459a0. [DOI] [PubMed] [Google Scholar]
- Munroe J. S. Progesteroids as immunosuppressive agents. J Reticuloendothel Soc. 1971 Apr;9(4):361–375. [PubMed] [Google Scholar]
- Olding L. B., Oldstone M. B. Lymphocytes from human newborns abrogate mitosis of their mother's lymphocytes. Nature. 1974 May 10;249(453):161–162. doi: 10.1038/249161a0. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Haran-Ghera N. Prevention of induced leukaemia in mice by immunological inhibition of adenohypophysis. Nature. 1975 Mar 27;254(5498):334–335. doi: 10.1038/254334a0. [DOI] [PubMed] [Google Scholar]
- Powell A. E. Letter: Maternal lymphocytes: suppression by human chorionic gonadotropin. Science. 1974 May 24;184(4139):913–914. doi: 10.1126/science.184.4139.913. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Hallgren H. M., Yunis E. J. Depressed maternal lymphocyte response to phytohaemagglutinin in human pregnancy. Lancet. 1972 Apr 8;1(7754):769–771. doi: 10.1016/s0140-6736(72)90522-3. [DOI] [PubMed] [Google Scholar]
- Schwarz M. R. The mixed lymphocyte reaction: an in vitro test for tolerance. J Exp Med. 1968 May 1;127(5):879–890. doi: 10.1084/jem.127.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkauskas A. J., Wilson B. S., Dray D., Dodson M. Inversion of levels of human T and B cells in early pregnancy. Nature. 1975 Nov 27;258(5533):331–332. doi: 10.1038/258331a0. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Steele R. W., Vincent M. M., Hensen S. A., Bellanti J. A. Impaired in vitro cell-mediated immunity to rubella virus during pregnancy. N Engl J Med. 1973 Sep 20;289(12):604–606. doi: 10.1056/NEJM197309202891203. [DOI] [PubMed] [Google Scholar]
- Truman J. W., Riddiford L. M. Neuroendocrine control of ecdysis in silkmoths. Science. 1970 Mar 20;167(3925):1624–1626. doi: 10.1126/science.167.3925.1624. [DOI] [PubMed] [Google Scholar]
- Waltman S. R., Burde R. M., Berrios J. Prevention of corneal homograft rejection by estrogens. Transplantation. 1971 Feb;11(2):194–196. doi: 10.1097/00007890-197102000-00016. [DOI] [PubMed] [Google Scholar]


