Abstract
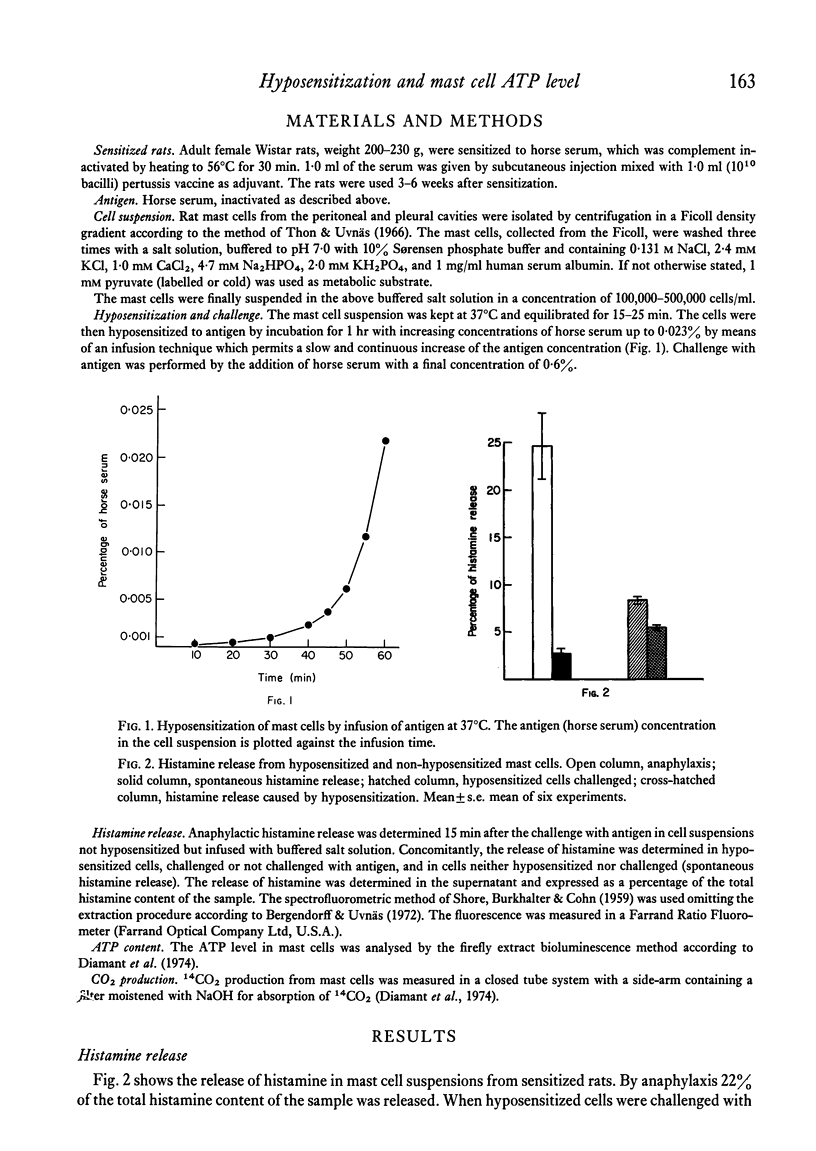
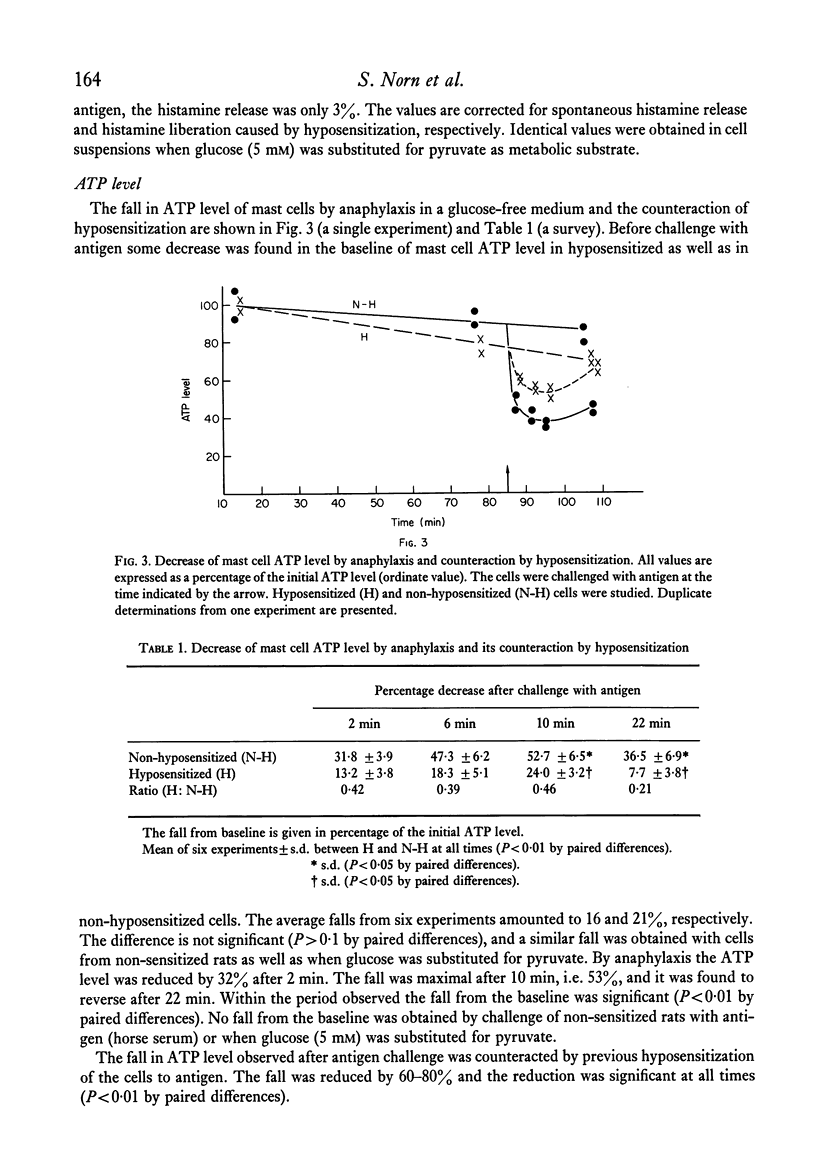
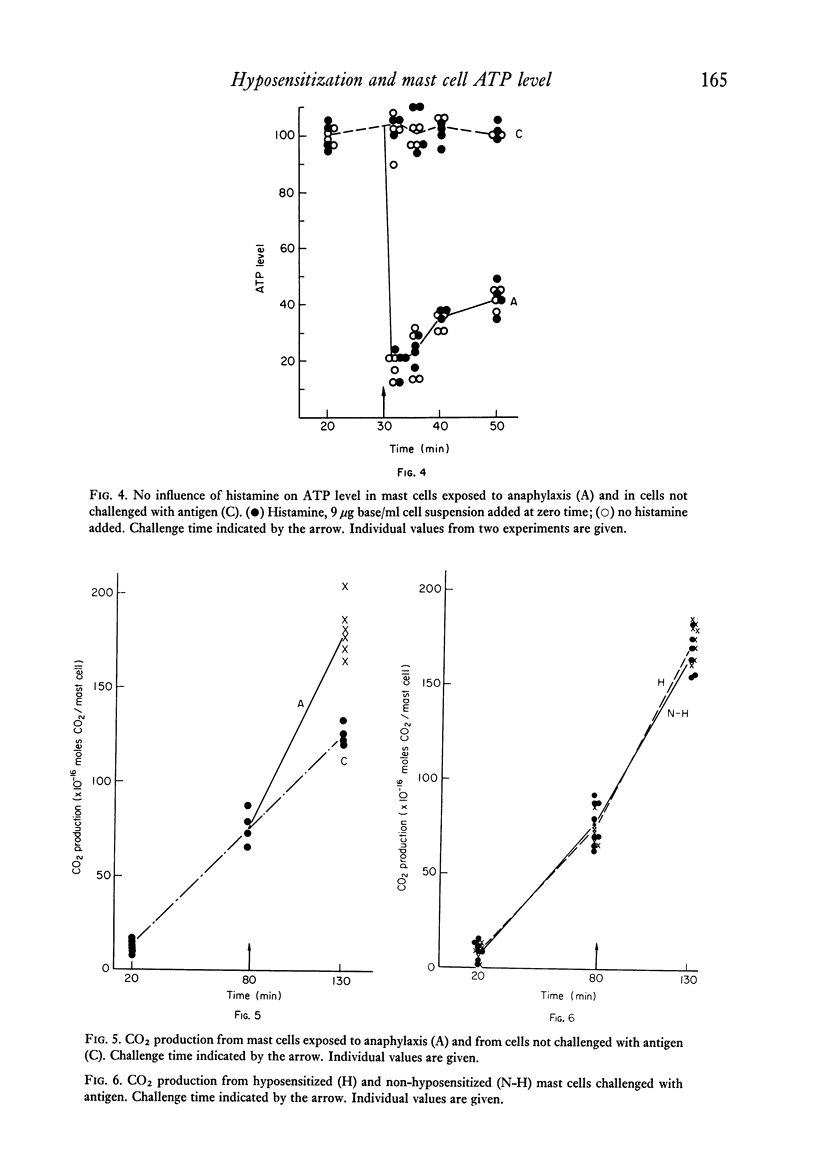
Anaphylaxis in a glucose-free medium containing pyruvate caused a release of histamine and a significant decrease in the ATP level of rat mast cells. The fall was maximal after 10 min and it was found to reverse after 22 min. Glucose completely counteracted the ATP fall without changing the anaphylactic histamine release. Furthermore, the oxidative metabolism of exogenous pyruvate to CO2 was stimulated in the mast cell. A high level of protection of mast cells to antigen challenge was obtained following hyposensitization and only a small amount of the intracellular histamine was released in contrast to non-hyposensitized cells. Hyposensitization counteracted the ATP fall by antigen challenge but the increase in oxidative metabolism remained unchanged. The results indicate that hyposensitization exerts effects in the mast cell consistent with a reduced ATP utilization or with a reduced uncoupling of oxidative phosphorylation. The mechanism of the hyposensitization must be due to inhibition of one or more of the cellular steps leading to histamine release and subsequent morphological changes of the cell or to uncoupling of oxidative phosphorylation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Slorach S. A., Uvnäs B. Sequential exocytosis of storage granules during antigen-induced histamine release from sensitized rat mast cells in vitro. An electron microscopic study. Acta Physiol Scand. 1973 Jul;88(3):359–372. doi: 10.1111/j.1748-1716.1973.tb05465.x. [DOI] [PubMed] [Google Scholar]
- Bergendorff A., Uvnäs B. Storage of 5-hydroxytryptamine in rat mast cells. Evidence for an ionic binding to carboxyl groups in a granule heparin-protein complex. Acta Physiol Scand. 1972 Mar;84(3):320–331. doi: 10.1111/j.1748-1716.1972.tb05183.x. [DOI] [PubMed] [Google Scholar]
- Bloom G. D., Chakravarty N. Time course of anaphylactic histamine release and morphological changes in rat peritoneal mast cells. Acta Physiol Scand. 1970 Mar;78(3):410–419. doi: 10.1111/j.1748-1716.1970.tb04677.x. [DOI] [PubMed] [Google Scholar]
- Diamant B. Energy production in rat mast cells and its role for histamine release. Int Arch Allergy Appl Immunol. 1975;49(1-2):155–171. doi: 10.1159/000231391. [DOI] [PubMed] [Google Scholar]
- Diamant B., Norn S., Felding P., Olsen N., Ziebell A., Nissen J. ATP level and CO2 production of mast cells in anaphylaxis. Int Arch Allergy Appl Immunol. 1974;47(6):894–908. doi: 10.1159/000231280. [DOI] [PubMed] [Google Scholar]
- Diamant B. The effects of compound 48-80 and distilled water on the adenosine triphosphate content of isolated rat mast cells. Acta Physiol Scand. 1967 Dec;71(4):283–290. doi: 10.1111/j.1748-1716.1967.tb03735.x. [DOI] [PubMed] [Google Scholar]
- Diamant B. The influence of adenosine triphosphate on isolated rat peritoneal mast cells. Int Arch Allergy Appl Immunol. 1969;36(1):3–21. doi: 10.1159/000230715. [DOI] [PubMed] [Google Scholar]
- Johansen T., Chakravarty N. The utilization of adenosine triphosphate in rat mast cells during histamine release induced by anaphylactic reaction and compound 48/80. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(2-3):243–260. doi: 10.1007/BF00500530. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Ishizaka K., Norman P. S., Sobotka A. K., Hill B. M. IgE antibody measurements in ragweed hay fever. Relationship to clinical severity and the results of immunotherapy. J Clin Invest. 1973 Feb;52(2):472–482. doi: 10.1172/JCI107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L. Clinical and in vitro studies on the role of immunotherapy in ragweed hay fever. Am J Med. 1968 Apr;44(4):514–524. doi: 10.1016/0002-9343(68)90052-1. [DOI] [PubMed] [Google Scholar]
- Norn S., Skov P. S. Anaphylactic hyposensitization of rat mast cells in vitro by antigen. Clin Exp Immunol. 1974 Nov;18(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Peterson C., Diamant B. Increased utilization of endogenous ATP in isolated rat mast cells during histamine release induced by compound 48-80. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):337–346. doi: 10.1111/j.1600-0773.1974.tb03530.x. [DOI] [PubMed] [Google Scholar]
- Peterson C. Histamine release induced by compound 48-80 from isolated rat cells: dependence on endogenous ATP. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):356–367. doi: 10.1111/j.1600-0773.1974.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Peterson C. Inhibitory action of antimycin A on histamine release from isolated rat mast cells. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):347–355. doi: 10.1111/j.1600-0773.1974.tb03531.x. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Sadan N., Rhyne M. B., Mellits E. D., Goldstein E. O., Levy D. A., Lichtenstein L. M. Immunotherapy of pollinosis in children: investigation of the immunologic basis of clinical improvement. N Engl J Med. 1969 Mar 20;280(12):623–627. doi: 10.1056/NEJM196903202801201. [DOI] [PubMed] [Google Scholar]
- Thon I. L., Uvnäs B. Mode of storage of histamine in mast cells. Acta Physiol Scand. 1966 Jul-Aug;67(3):455–470. doi: 10.1111/j.1748-1716.1966.tb03332.x. [DOI] [PubMed] [Google Scholar]


