Abstract
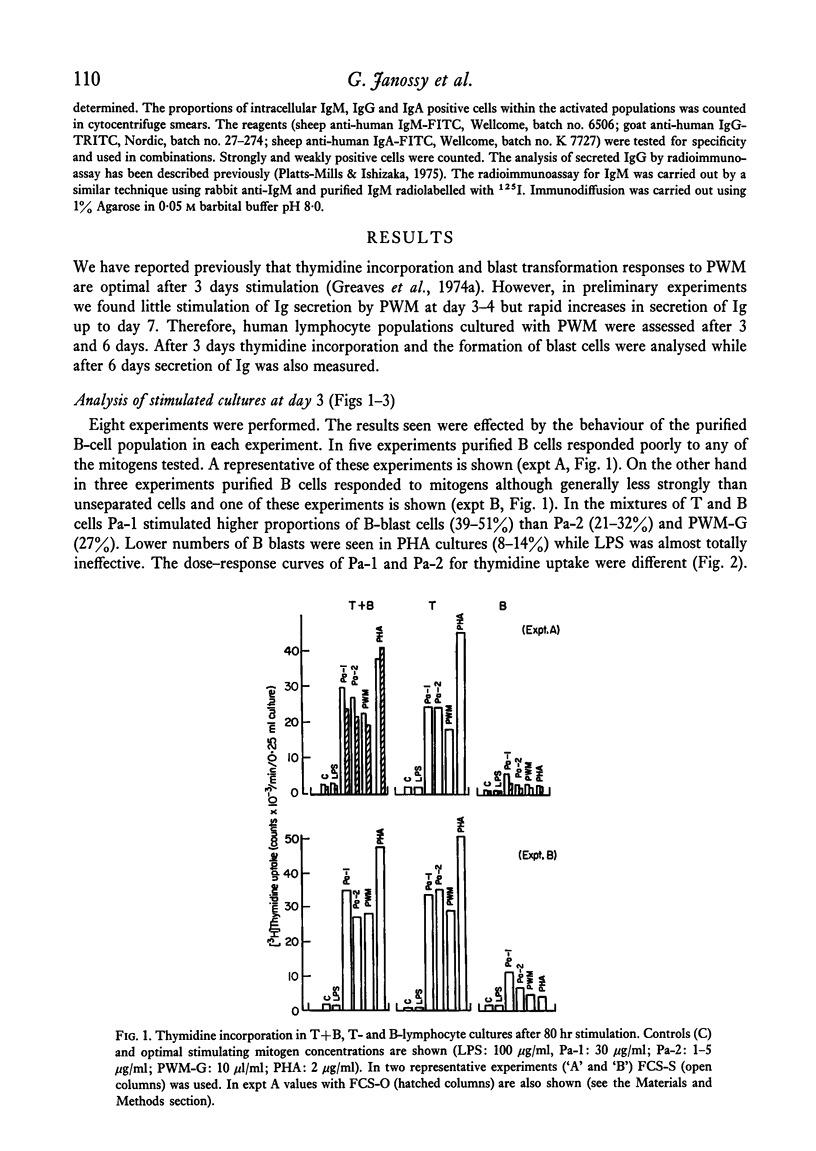
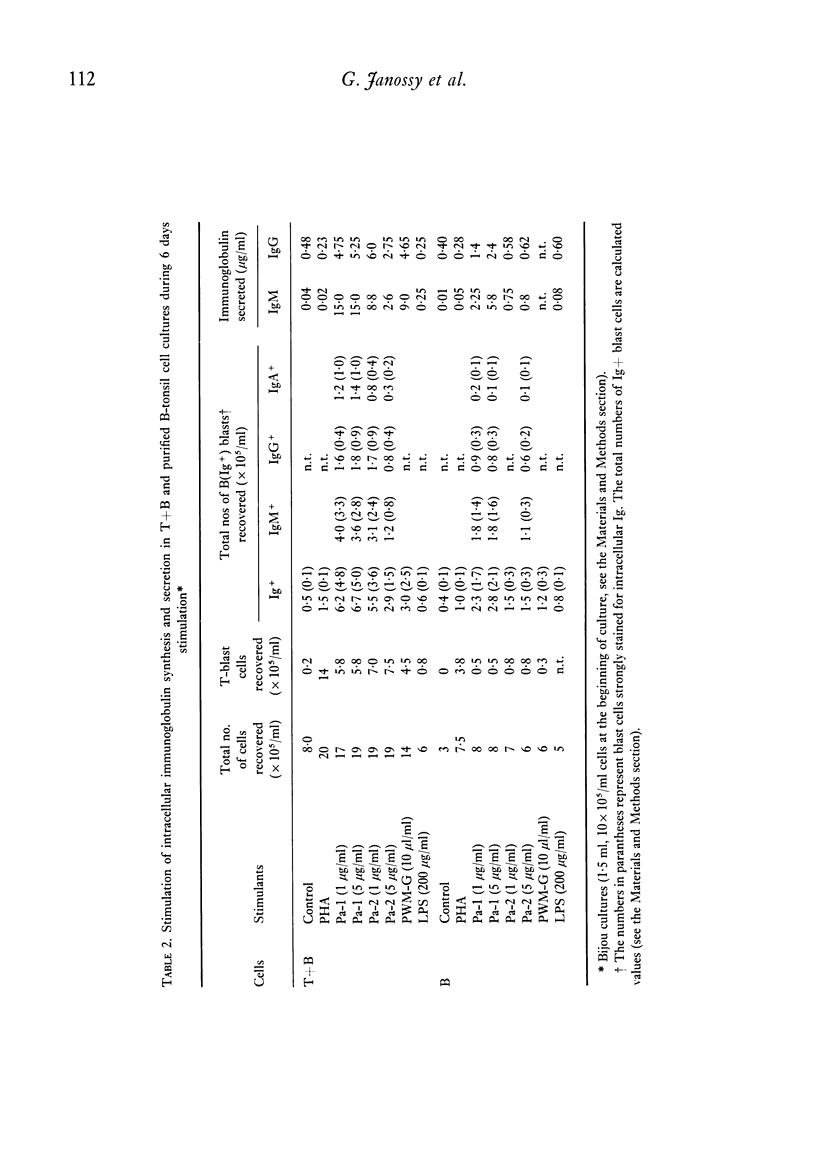
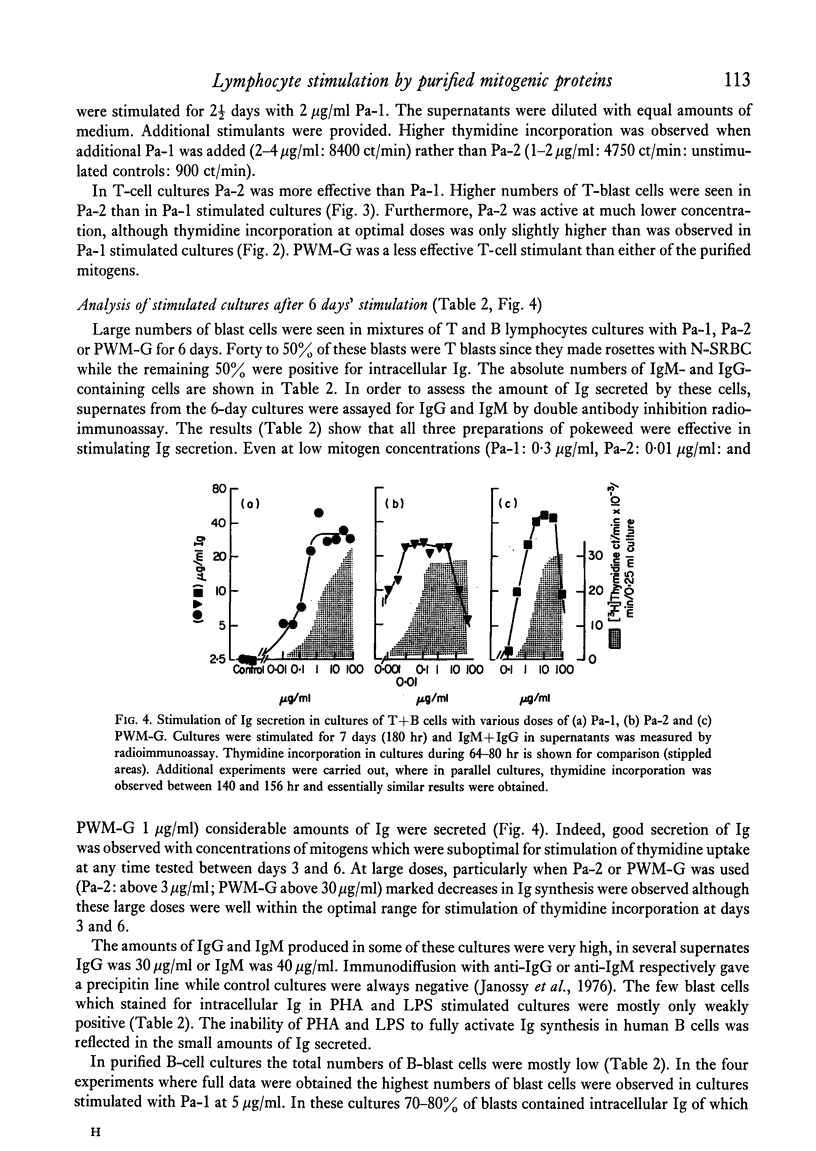
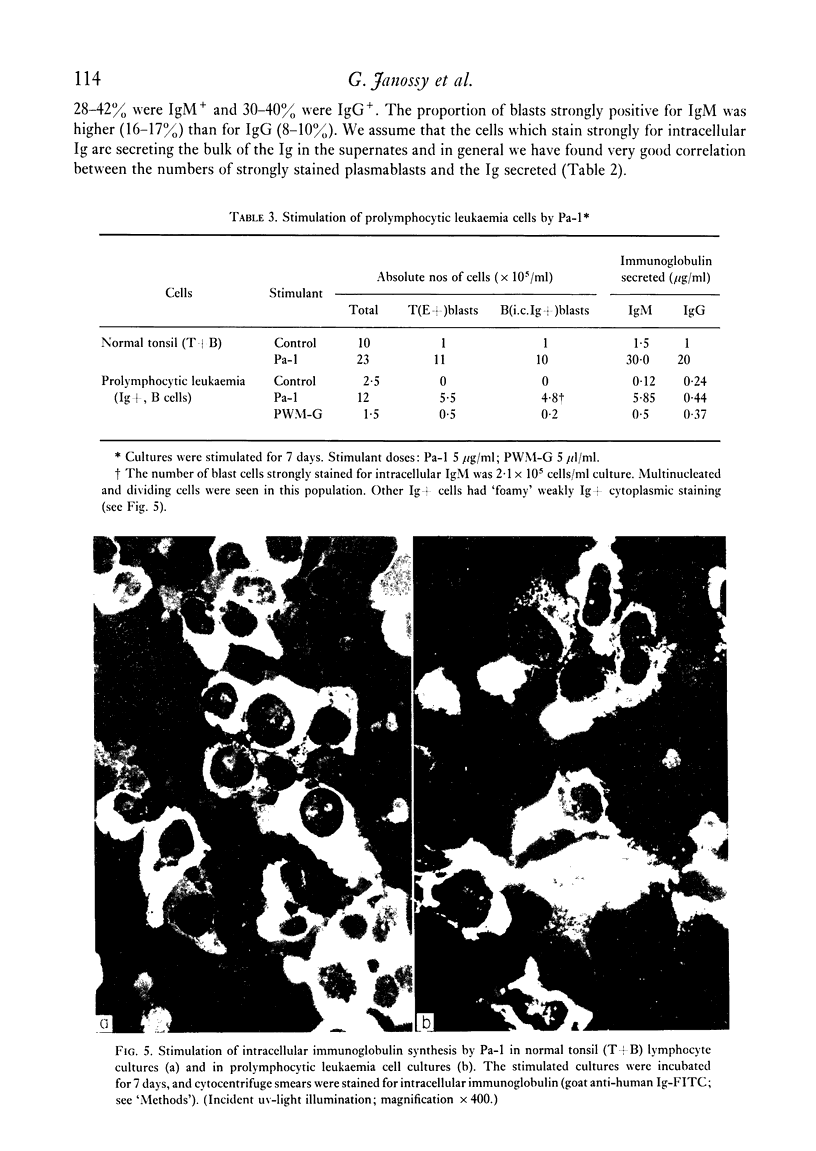
Purified proteins (Pa-1 and Pa-2) from pokeweed have been compared with commercial pokeweed mitogen (PWM-G) and other mitogens in their ability to stimulate human lymphocytes. With cultures of T and B cells separated from tonsil lymphocytes, thymidine uptake, blast transformation and immunoglobulin (Ig) synthesis have been measured. IgM and IgG was measured in supernates of stimulated cultures by radioimmunoassay. Pa-1, Pa-2 and PWM-G were found to be potent mitogens for unseparated tonsil lymphocytes or nylon column purified T cells. Pa-2 was found to be active at lower concentrations than Pa-1, and PWM-G was less potent than the purified mitogens. These three mitogens all stimulated unseparated lymphocytes to secrete large quantities of Ig (20-100 mug/ml) during 7 days in culture. With increasing amounts of mitogens severe decreases in immunoglobulin synthesis were observed at day 6 even with doses which were still optimal for stimulation of thymidine uptake at days 3 and 6. With purified B cells (less than 2% T cells) Pa-1 was the best mitogen for thymidine incorporation. However, the secretory response was very variable. In some experiments B cells did not secrete Ig in response to mitogens; in others Pa-1 was clearly more effective at stimulating secretion than Pa-2 or PWM-G and in some experiments B cells were stimulated by all three. In one experiment Pa-1 stimulated prolymphocytic leukaemia cells to blast transformation and the secretion of IgM. It is concluded that Pa-1, Pa-2 and PWM-G are much better activators of Ig synthesis in human cultures than either PHA or LPS and that Pa-1 is the most reliable B-cell stimulant of the three.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basham T. Y., Waxdal M. J. The stimulation of immunoglobulin production in murine spleen cells by the pokeweed mitogens. J Immunol. 1975 Feb;114(2 Pt 1):715–716. [PubMed] [Google Scholar]
- Broom B. C., de la Concha E. G., Webster A. D., Loewi G., Asherson G. L. Dichotomy between immunoglobulin synthesis by cells in gut and blood of patients with hypogammaglobulinaemia. Lancet. 1975 Aug 9;2(7928):253–256. doi: 10.1016/s0140-6736(75)90965-4. [DOI] [PubMed] [Google Scholar]
- Chessin L. N., Börjeson J., Welsh P. D., Douglas S. D., Cooper H. L. Studies on human peripheral blood lymphocytes in vitro. II. Morphological and biochemical studies on the transformation of lymphocytes by pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):873–884. doi: 10.1084/jem.124.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohnen G., Douglas S. D., König E., Brittinger G. Pokeweed mitogen response of lymphocytes in chronic lymphocytes in chronic lymphocytic leukemia: a fine structural study. Blood. 1973 Oct;42(4):591–600. [PubMed] [Google Scholar]
- Doenhoff M. J., Janossy G., Greaves M. F., Gomer K. J., Snajdr J. Lymphocyte activation. VI. A re-evaluation of factors affecting the selectivity of polyclonal mitogens for mouse T and B cells. Clin Exp Immunol. 1974 Jul;17(3):475–490. [PMC free article] [PubMed] [Google Scholar]
- Douglas S. D., Kamin R. M., Fudenberg H. H. Human lymphocyte response to phytomitogens in vitro: normal, agammaglobulinemic and paraproteinemic individuals. J Immunol. 1969 Dec;103(6):1185–1195. [PubMed] [Google Scholar]
- Galili U., Schlesinger M. The formation of stable E rosettes after neuraminidase treatment of either human peripheral blood lymphocytes or of sheep red blood cells. J Immunol. 1974 May;112(5):1628–1634. [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Greaves M., Janossy G., Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974 Jul 1;140(1):1–18. doi: 10.1084/jem.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Functional analysis of B cell heterogeneity. Transplant Rev. 1975;24:3–40. doi: 10.1111/j.1600-065x.1975.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Hijmans W., Schuit H. R., Klein F. An immunofluorescence procedure for the detection of intracellular immunoglobulins. Clin Exp Immunol. 1969 Apr;4(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. Functional analysis of murine and human B lymphocyte subsets. Transplant Rev. 1975;24:177–236. doi: 10.1111/j.1600-065x.1975.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Jones G. Lymphocyte activation. I. Expression of theta, H-2 and immunoglobulin determinants on lymphocytes stimulated by phytohaemagglutinin, pokeweed mitogen, concanavalin A or histocompatibility antigen. Clin Exp Immunol. 1972 Nov;12(3):391–402. [PMC free article] [PubMed] [Google Scholar]
- Mellstedt H., Jondal M., Holm G. In vitro studies of lymphocytes from patients with plasma cell myeloma. II. Characterization by cell surface markers. Clin Exp Immunol. 1973 Nov;15(3):321–330. [PMC free article] [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. IgA and IgA diphtheria antitoxin responses from human tonsil lymphocytes. J Immunol. 1975 Mar;114(3):1058–1064. [PubMed] [Google Scholar]
- Scher I., Strong D. M., Ahmed A., Knudsen R. C., Sell K. W. Specific murine B-cell activation by synthetic single-and double-stranded polynucleotides. J Exp Med. 1973 Dec 1;138(6):1545–1563. doi: 10.1084/jem.138.6.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Cowling D. C., Barker C. R. Response of lymphocytes in chronic lymphocytic leukaemia to plant mitogens. Lancet. 1972 Jan 29;1(7744):229–233. doi: 10.1016/s0140-6736(72)90624-1. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Waxdal M. J., Basham T. Y. B and T-cell stimulatory activities of multiple mitogens from pokeweed. Nature. 1974 Sep 13;251(5471):163–164. doi: 10.1038/251163a0. [DOI] [PubMed] [Google Scholar]
- Waxdal M. J. Isolation, characterization, and biological activities of five mitogens from pokeweed. Biochemistry. 1974 Aug 27;13(18):3671–3677. doi: 10.1021/bi00715a008. [DOI] [PubMed] [Google Scholar]
- Weber W. T. Direct evidence for the response of B and T cells to pokeweed mitogen. Cell Immunol. 1973 Dec;9(3):482–487. doi: 10.1016/0008-8749(73)90064-6. [DOI] [PubMed] [Google Scholar]
- Wu L. Y., Lawton A. R., Cooper M. D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973 Dec;52(12):3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]


