Abstract
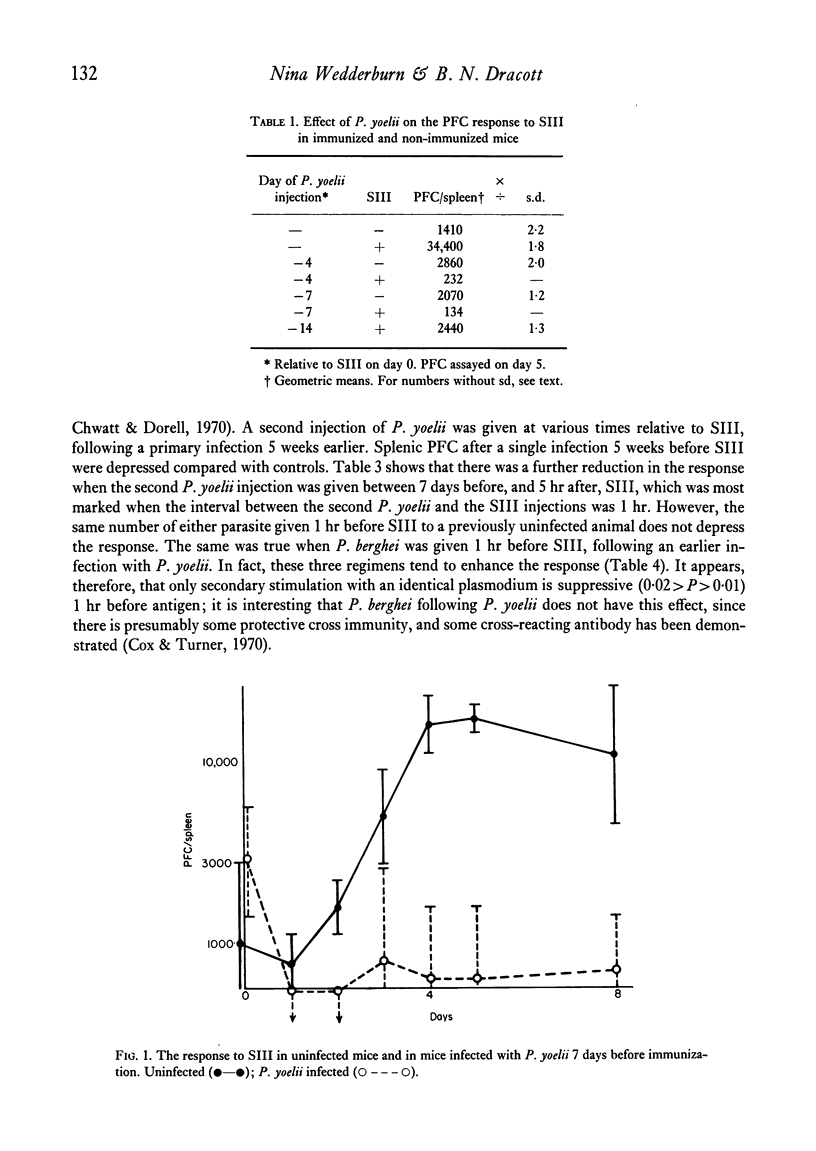
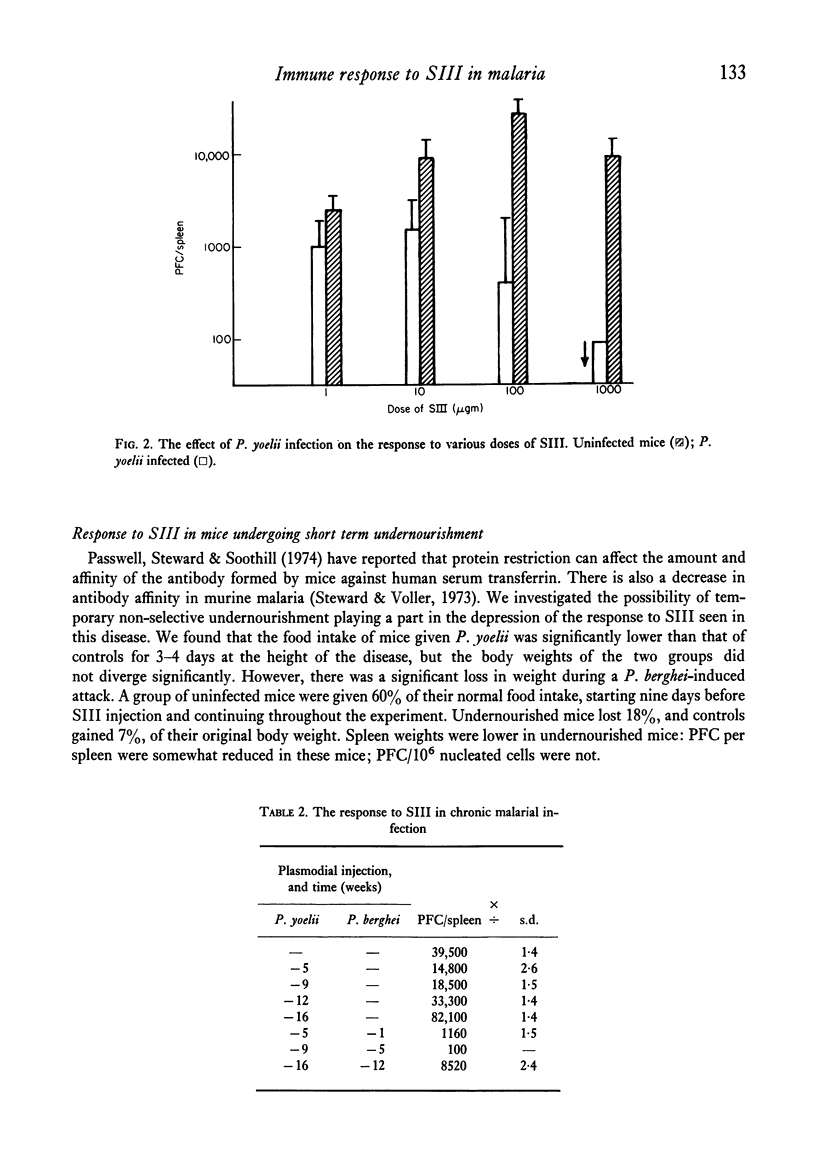
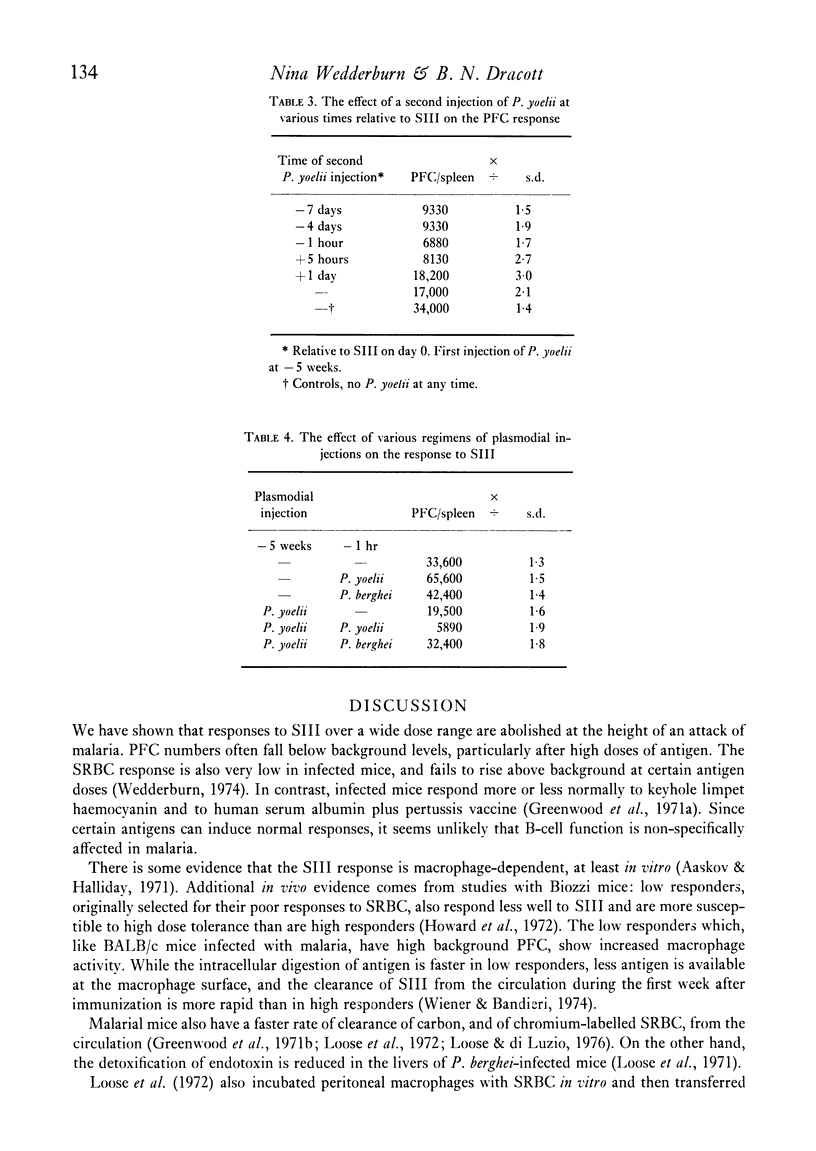
The immune response of BALB/c mice to type III pneumococcal polysaccharide (SIII), as measured by splenic PFC, was abolished at the height of an acute self-limiting attack of malaria caused by the murine plasmodium P. yoelii, over a wide range of antigen doses. The response to antigen, given at various times after clinical recovery, gradually reappeared, but did not reach normal levels until 12 weeks after the injection of the parasite. A second injection of P. yoelii given 1 hr before SIII caused a moderate degree of depression, although in this case the plasmodium does not multiply. In chronic malaria the response to SIII was also very poor. Short term under-nourishment was found to reduce only slightly the response to SIII.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., FARTHING C. P., HUMPHREY J. H. The significance of multiple antibody components in serum of immunized rabbits. Immunology. 1960 Oct;3:336–351. [PMC free article] [PubMed] [Google Scholar]
- Aaskov J. G., Halliday W. J. Requirement for lymphocyte-macrophage interaction in the response of mouse spleen cultures to pneumococcal polysaccharide. Cell Immunol. 1971 Aug;2(4):335–340. doi: 10.1016/0008-8749(71)90068-2. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Baker P. J., Prescott B. Letter: The basis for conflicting results obtained in studies on the plaque-forming cell response to type III pneumococcal polysaccharide. J Immunol. 1975 Sep;115(3):891–892. [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B., Barth R. F. Evidence for the existence of two functionally distinct types of cells which regulate the antibody response to type 3 pneumococcal polysaccharide. J Immunol. 1970 Dec;105(6):1581–1583. [PubMed] [Google Scholar]
- Barker L. R. Experimental malaria: effects upon the immune response to different antigens. J Infect Dis. 1971 Jan;123(1):99–101. doi: 10.1093/infdis/123.1.99. [DOI] [PubMed] [Google Scholar]
- Byfield P., Christie G. H., Howard J. G. Alternative potentiating and inhibitory effects of GVH reaction on formation of antibodies against a thymus-independent polysaccharide (S3). J Immunol. 1973 Jul;111(1):72–84. [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cox F. E., Turner S. A. Antigenic relationships between the malaria parasites and piroplasms of mice as determined by the fluorescent-antibody technique. Bull World Health Organ. 1970;43(2):337–340. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Brown J. C., De Jesus D. G., Holborow E. J. Immunosuppression in murine malaria. II. The effect on reticulo-endothelial and germinal centre function. Clin Exp Immunol. 1971 Sep;9(3):345–354. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Playfair J. H., Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971 Mar;8(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M., Biozzi G. Studies on immunological paralysis. 8. Pneumococcal polysaccharide tolerance and immunity differences between the Biozzi high and low responder lines of mice. Eur J Immunol. 1972 Jun;2(3):269–273. doi: 10.1002/eji.1830020315. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M., Leuchars E., Davies A. J. Studies on immunological paralysis. VI. Thymic-independence of tolerance and immunity to type 3 pneumococcal polysaccharide. Cell Immunol. 1971 Dec;2(6):614–626. doi: 10.1016/0008-8749(71)90009-8. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M. Studies on immunological paralysis. IV. The relative contributions of continuous antibody neutralization and central inhibition to paralysis with type 3 pneumococcal polysaccharide. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):417–438. doi: 10.1098/rspb.1971.0073. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. Parasitic protozoa of the blood of rodents: a revision of Plasmodium berghei. Parasitology. 1974 Oct;69(2):225–237. doi: 10.1017/s0031182000048071. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Nussenzweig R. Depletion of T and B lymphocytes during malarial infections. Cell Immunol. 1974 Sep;13(3):440–446. doi: 10.1016/0008-8749(74)90263-9. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Shiozawa C., Shaw A., Diener E. Requirement for accessory cells in the antibody response to T cell-independent antigens in vitro. Eur J Immunol. 1976 Jan;6(1):63–68. doi: 10.1002/eji.1830060114. [DOI] [PubMed] [Google Scholar]
- Loose L. D., Trejo R., Di Luzio N. R. Impaired endotoxin detoxification as a factor in enhanced endotoxin sensitivity of malaria infected mice. Proc Soc Exp Biol Med. 1971 Jul;137(3):794–797. doi: 10.3181/00379727-137-35669. [DOI] [PubMed] [Google Scholar]
- Loose L. D., di Luzio N. R. A temporal relationship between reticuloendothelial system phagocytic alterations and antibody responses in mice infected with Plasmodium berghei (NYU-2 strain). Am J Trop Med Hyg. 1976 Mar;25(2):221–228. doi: 10.4269/ajtmh.1976.25.221. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Passwell J. H., Steward M. W., Soothill J. F. The effects of protein malnutrition on macrophage function and the amount and affinity of antibody response. Clin Exp Immunol. 1974 Jul;17(3):491–495. [PMC free article] [PubMed] [Google Scholar]
- Salaman M. H., Wedderburn N., Bruce-Chwatt L. J. The immunodepressive effect of a murine plasmodium and its interaction with murine oncogenic viruses. J Gen Microbiol. 1969 Dec;59(3):383–391. doi: 10.1099/00221287-59-3-383. [DOI] [PubMed] [Google Scholar]
- Sengers R. C., Jerusalem C. R., Doesburg W. H. Murine malaria. IV. Disturbed immunological responsiveness during Plasmodium berghei infection. Exp Parasitol. 1971 Aug;30(1):41–53. doi: 10.1016/0014-4894(71)90068-3. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Voller A. The effect of malaria on the relative affinity of mouse antiprotein antibody. Br J Exp Pathol. 1973 Apr;54(2):198–202. [PMC free article] [PubMed] [Google Scholar]
- Topley E., Bruce-Chwatt L. J., Dorrell J. Haematological study of a rodent malaria model. J Trop Med Hyg. 1970 Jan;73(1):1–8. [PubMed] [Google Scholar]
- Voller A., Gall D., Manawadu B. R. Depression of the antibody response to tetanus toxoid in mice infected with malaria parasites. Z Tropenmed Parasitol. 1972 Jun;23(2):152–155. [PubMed] [Google Scholar]
- Warr G. W., Ghaffar A., James K. The response of mice to type III pneumococcal polysaccharide: failure to detect thymus-derived suppressor cells. Cell Immunol. 1975 Jun;17(2):366–373. doi: 10.1016/s0008-8749(75)80040-2. [DOI] [PubMed] [Google Scholar]
- Wedderburn N., Turk J. L., Hutt M. S. Chronic malarial infection in Balb/C mice. Effect on the immune response to sheep erythrocytes and histological changes in the liver and spleen. Trans R Soc Trop Med Hyg. 1975;69(5-6):468–472. doi: 10.1016/0035-9203(75)90099-1. [DOI] [PubMed] [Google Scholar]
- Wiener E., Bandieri A. Differences in antigen handling by peritoneal macrophages from the Biozzi high and low responder lines of mice. Eur J Immunol. 1974 Jul;4(7):457–463. doi: 10.1002/eji.1830040703. [DOI] [PubMed] [Google Scholar]


