Abstract
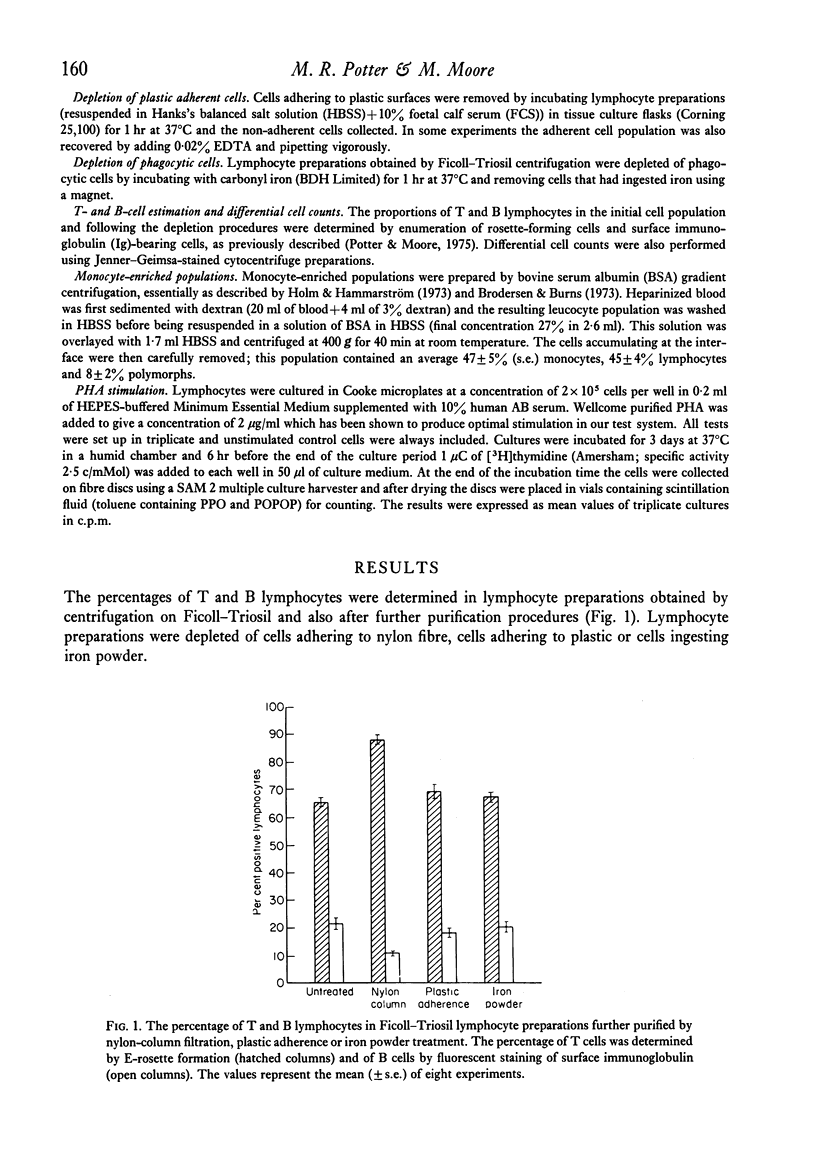
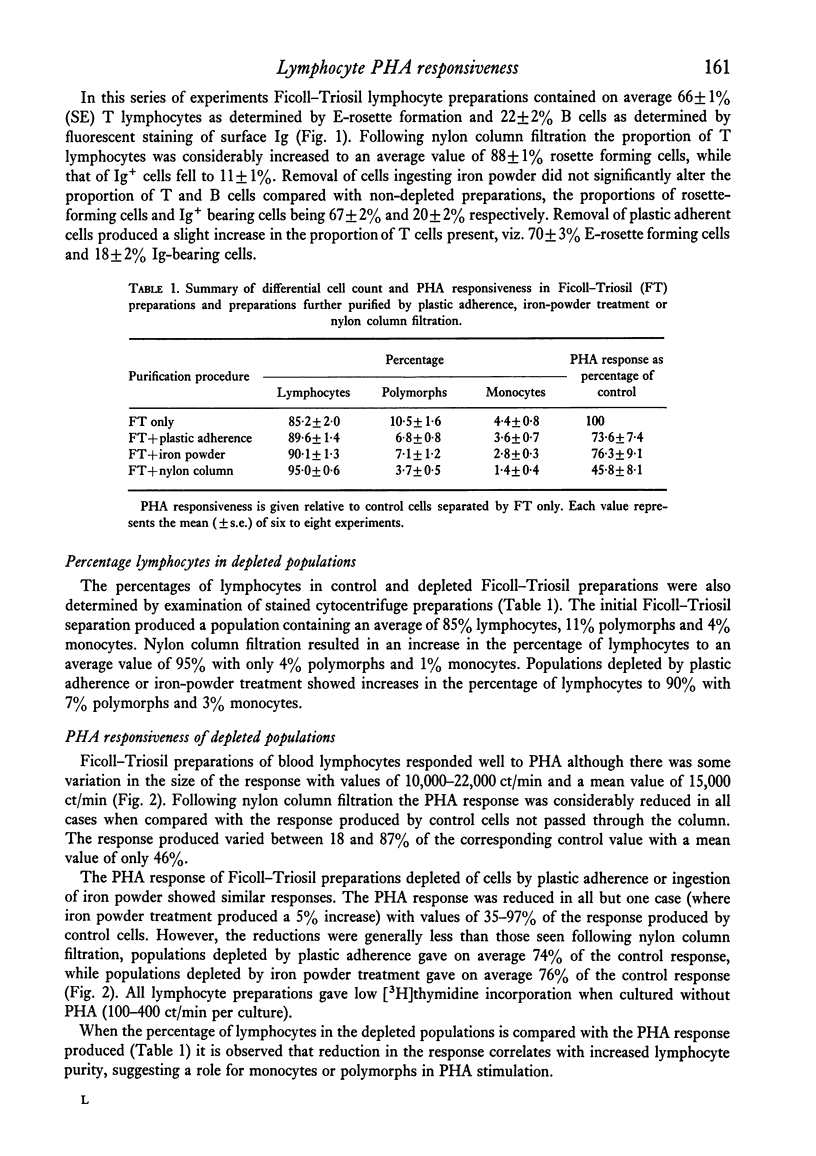
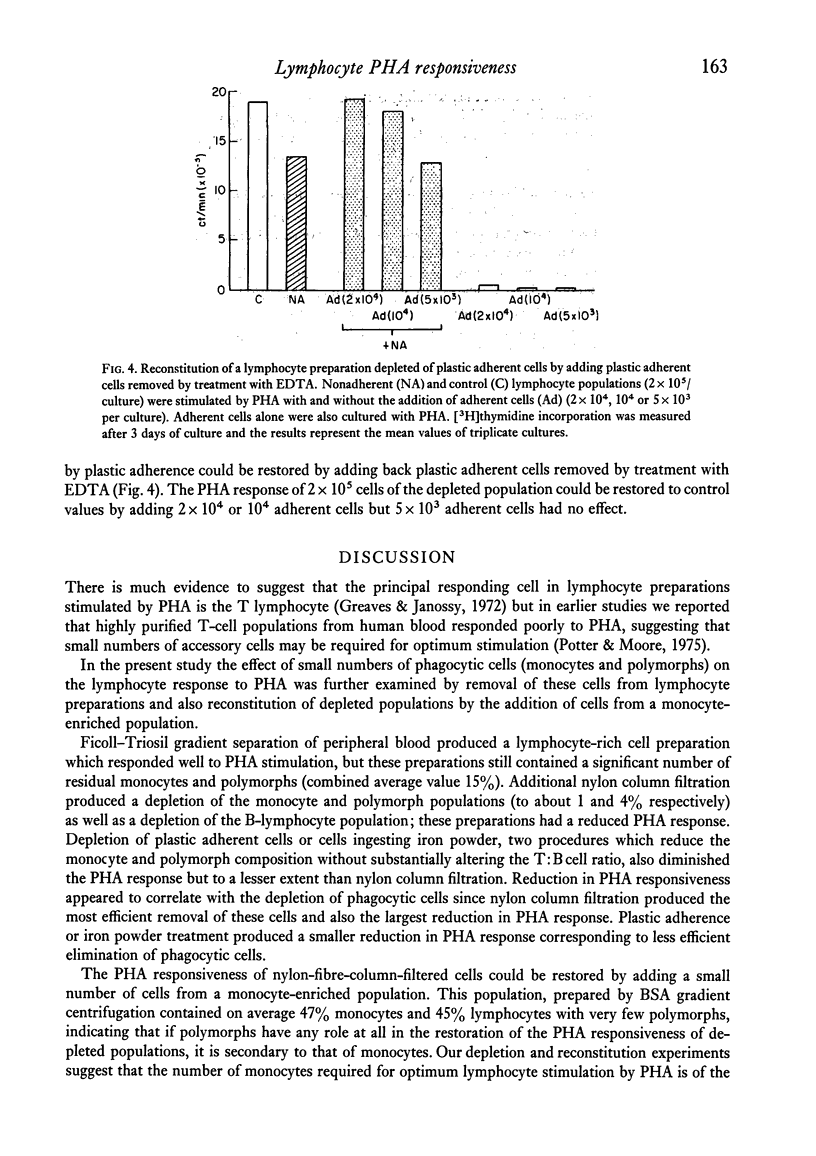
The effect of small numbers of adherent and phagocytic cells on the human peripheral blood lymphocyte response to PHA was examined by depleting these cells from lymphocyte preparations. Lymphocyte preparations obtained by centrifugation on Ficoll--Triosil, which contained on average 85% lymphocytes, responded well to PHA. Depletion of cells adhering to nylon fibre, giving a population containing on average 95% lymphocytes, resulted in a considerably reduced response. Depletion of cells that adhered to plastic or ingested iron powder to give populations containing on average 90% lymphocytes, also reduced the PHA response, but to a lesser extent. Reduction in PHA responsiveness correlated with increasing lymphocyte purity. The responsiveness of nylon-column-filtered cells could be restored by adding a small number of cells from a monocyte-rich population.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodersen M. P., Burns C. P., Hoak J. C. The separation of human monocytes from blood including biochemical observations. Proc Soc Exp Biol Med. 1973 Dec;144(3):941–944. doi: 10.3181/00379727-144-37716. [DOI] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Cell surface markers for human T and B lymphocytes. Eur J Immunol. 1974 Apr;4(4):302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- Folch H., Yoshinaga M., Waksman B. H. Regulation of lymphocyte responses in vitro. 3. Inhibition by adherent cells of the T-lymphocyte response to phytohemagglutinin. J Immunol. 1973 Mar;110(3):835–839. [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Hedfors E., Holm G., Pettersson D. Activation of human peripheral blood lymphocytes by concanavalin A dependence of monocytes. Clin Exp Immunol. 1975 Nov;22(2):223–229. [PMC free article] [PubMed] [Google Scholar]
- Holm G., Hammarström S. Haemolytic activity of human blood monocytes. Lysis of human erythrocytes treated with anti-A serum. Clin Exp Immunol. 1973 Jan;13(1):29–43. [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Levis W. R., Robbins J. H. Effect of glass-adherent cells on the blastogenic response of 'purified' lymphocytes to phytohemagglutinin. Exp Cell Res. 1970 Jul;61(1):153–158. doi: 10.1016/0014-4827(70)90269-7. [DOI] [PubMed] [Google Scholar]
- Levis W. R., Robbins J. H. Methods for obtaining purified lymphocytes, glass-adherent mononuclear cells, and a population containing both cell types from human peripheral blood. Blood. 1972 Jul;40(1):77–89. [PubMed] [Google Scholar]
- Potter M. R., Moore M. PHA stimulation of separated human lymphocyte populations. Clin Exp Immunol. 1975 Sep;21(3):456–467. [PMC free article] [PubMed] [Google Scholar]


