Abstract
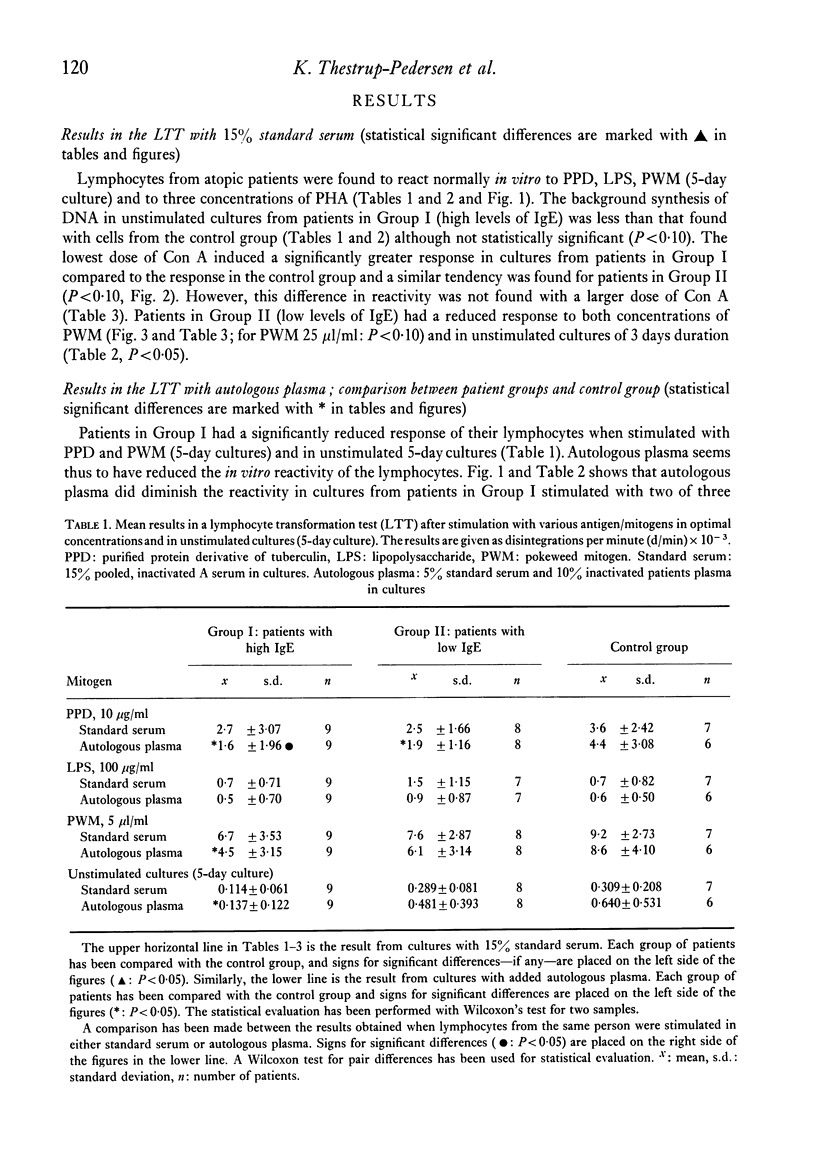
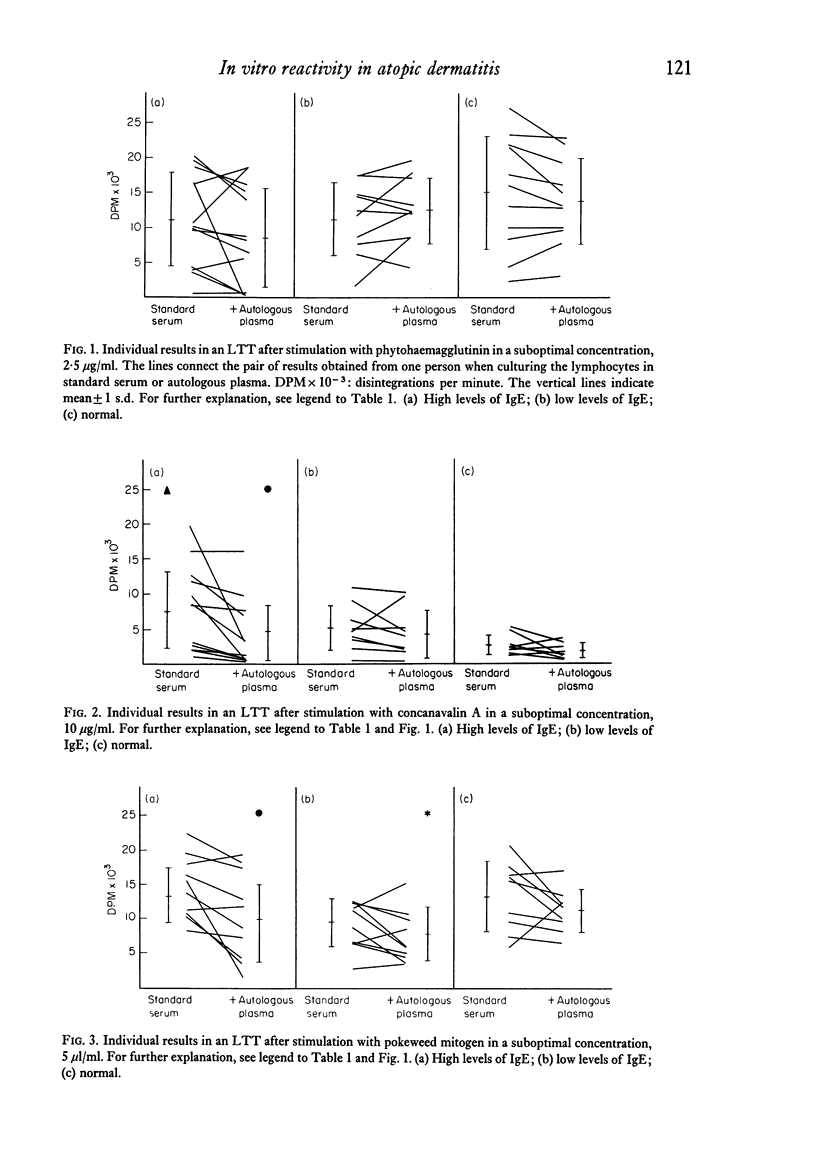
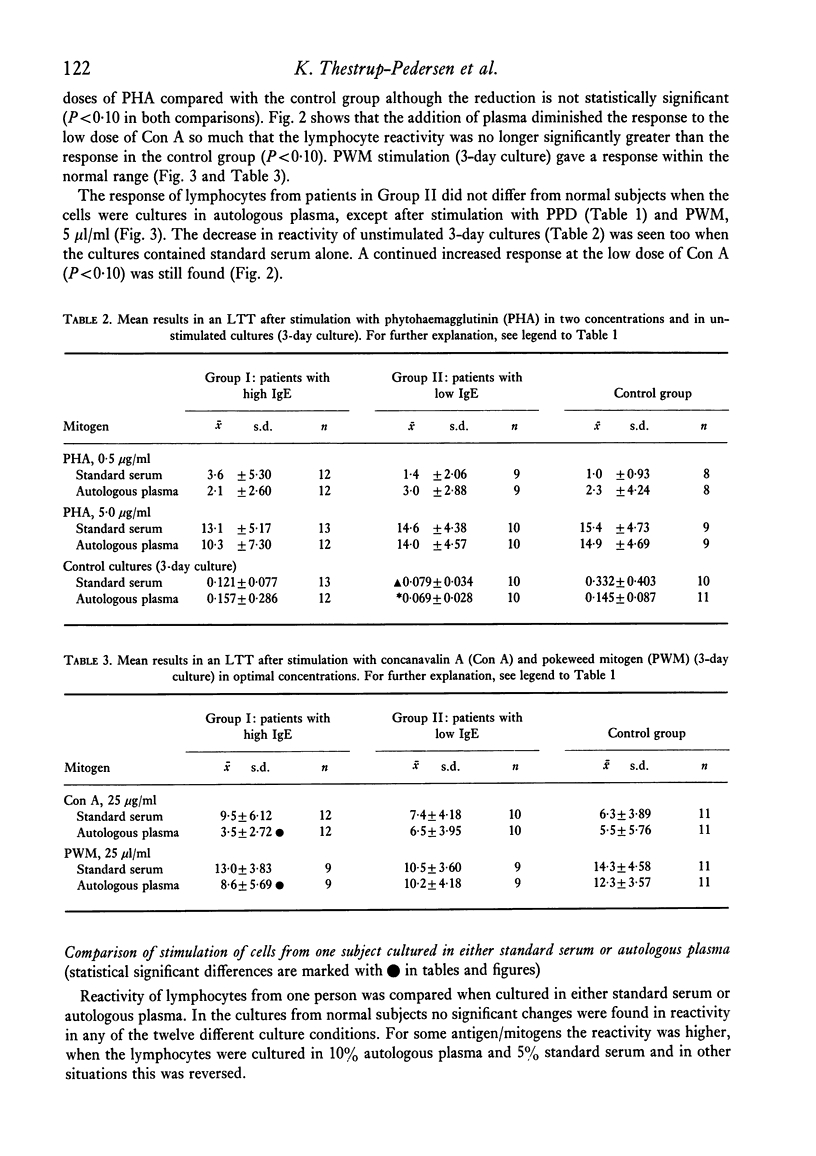
Lymphocytes from twenty-five patients with atopic dermatitis were investigated for their in vitro reactivity to stimulation with tuberculin (PPD), lipopolysaccharide (LPS), phytohaemagglutinin (PHA), concanavalin A (Con A) and pokeweed mitogen (PWM). The response to a low dose of Con A was increased, and the reactivity in unstimulated cultures tended to be lower than similar cultures from the control group. Addition of inactivated autologous plasma to the cultures had an inhibitory effect, when the plasma came from patients with high levels of IgE. The patients' in vitro reactivity to PPD in a leucocyte migration test was equal to that found in normal persons and no effect was observed after addition of autologous serum. The mean percentage of E rosette forming cells was significantly reduced in patients with high levels of IgE. The number of EAC rosette forming cells was within normal range. It is hypothesized that the observations could reflect the existence of suppressor mechanisms in patients where the immune system is strongly stimulated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen E., Hjorth N. B lymphocytes, T lymphocytes and phytohaemagglutinin responsiveness in atopic dermatitis. Acta Derm Venereol. 1975;55(5):345–349. [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Clausen J. E. Leukocyte migration agarose technique: some technical details. Acta Allergol. 1973 Dec;28(5):351–364. doi: 10.1111/j.1398-9995.1973.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. G. The establishment of a normal human population dose-response curve for lymphocytes cultured with PHA (phytohaemagglutinin). Clin Exp Immunol. 1971 Mar;8(3):421–425. [PMC free article] [PubMed] [Google Scholar]
- Identification, enumeration, and isolation of B and T lymphocytes from human peripheral blood. Report of a WHO-IARC-sponsored workshop on human B and T cells, London, 15-17 July 1974. Scand J Immunol. 1974;3(5):521–532. [PubMed] [Google Scholar]
- JOHNSON H. H., Jr, DEOREO G. A., LASCHEID W. P., MITCHELL F. Skin histamine levels in chronic atopic dermatitis. J Invest Dermatol. 1960 Apr;34:237–238. [PubMed] [Google Scholar]
- Jacobsson H., Blomgren H. Responses of mouse thymic cells to mitogens. A comparison between phytohaemagglutinin and concanavalin A. Cell Immunol. 1974 Mar 30;11(1-3):427–441. doi: 10.1016/0008-8749(74)90041-0. [DOI] [PubMed] [Google Scholar]
- Johansson S. G., Bennich H., Wide L. A new class of immunoglobulin in human serum. Immunology. 1968 Feb;14(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E., Lewis C. W., McMarlin S. L. Allergic contact sensitivity in atopic dermatitis. Arch Dermatol. 1973 Feb;107(2):217–222. [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- Lobitz W. C., Jr, Honeyman J. F., Winkler N. W. Suppressed cell-mediated immunity in two adults with atopic dermatitis. Br J Dermatol. 1972 Apr;86(4):317–328. doi: 10.1111/j.1365-2133.1972.tb05045.x. [DOI] [PubMed] [Google Scholar]
- Luckasen J. R., Sabad A., Goltz R. W., Kersey J. H. T and B lymphocytes in atopic eczema. Arch Dermatol. 1974 Sep;110(3):375–377. [PubMed] [Google Scholar]
- Malavé I., Layrisse Z., Layrisse M. Dose-dependent hyporreactivity to phytohemagglutinin in systemic lupus erythematosus. Cell Immunol. 1975 Feb;15(2):231–236. doi: 10.1016/0008-8749(75)90001-5. [DOI] [PubMed] [Google Scholar]
- Mangi R. J., Dwyer J. M., Kantor F. S. The effect of plasma upon lymphocyte response in vitro. Demonstration of a humoral inhibitor in patients with sarcoidosis. Clin Exp Immunol. 1974 Dec;18(4):519–528. [PMC free article] [PubMed] [Google Scholar]
- Palacios J., Fuller E. W., Blaylock W. K. Immunological capabilities of patients with atopic dermatitis. J Invest Dermatol. 1966 Nov;47(5):484–490. doi: 10.1038/jid.1966.172. [DOI] [PubMed] [Google Scholar]
- Rawson A. J., Huang T. C. The response to phytohemagglutinin or to concanavalin A as a probe for subpopulations of human peripheral blood lymphocytes. Cell Immunol. 1975 May;17(1):310–314. doi: 10.1016/s0008-8749(75)80032-3. [DOI] [PubMed] [Google Scholar]
- Sampson D., Grotelueschen C., Kauffman H. M., Jr The human splenic suppressor cell. Transplantation. 1975 Nov;20(5):362–367. doi: 10.1097/00007890-197511000-00002. [DOI] [PubMed] [Google Scholar]
- Schöpf E., Böhringer D. Zell-vermittelte Immunität bei Neurodermitis atopica. Hautarzt. 1974 Sep;25(9):420–426. [PubMed] [Google Scholar]
- Tardieu M., Daguillard F. Fractionated T lymphocytes can inhibit mitogenic responses through dialyzable factors. Cell Immunol. 1975 Sep;19(1):148–150. doi: 10.1016/0008-8749(75)90300-7. [DOI] [PubMed] [Google Scholar]
- Thulin H., Thestrup-Pedersen K., Ellegaard J. In vitro parameters of cell-mediated immune reactions in healthy individuals following immune-stimulation attempts with levamisole. Acta Allergol. 1975 Apr;30(1):9–18. doi: 10.1111/j.1398-9995.1974.tb02692.x. [DOI] [PubMed] [Google Scholar]


