Abstract
A significant hyperplasia of the thymus was induced in mice, treated with triiodothyronine during the first month of life. Stereological data showed that, in both treated and control mice, mononucleate epithelial cells were four times more numerous in the medulla that in the cortex. After triiodothyronine treatment, their absolute number in both cortex and medulla increased two-fold. The number of thymic epithelial cells could thus be regulated by thyroid hormones. The cortical volume in treated mice was also twice that of controls but medullar volume showed and increase of only fifty percent. Cortical epithelial cells increased at the same rate of the cortex volume by medullary epithelial cells grew more rapidly. In fact the medullary volume enlargement could be be explained mainly by the growth of the epithelium. Medullary lymphocytes did thus not preliferate in the same way as cortical lymphocytes after thyroid hormone administration. The recently described multinucleate epithelial cells were not modified in number and were thus insensitive to thyroid hormones.
Full text
PDF

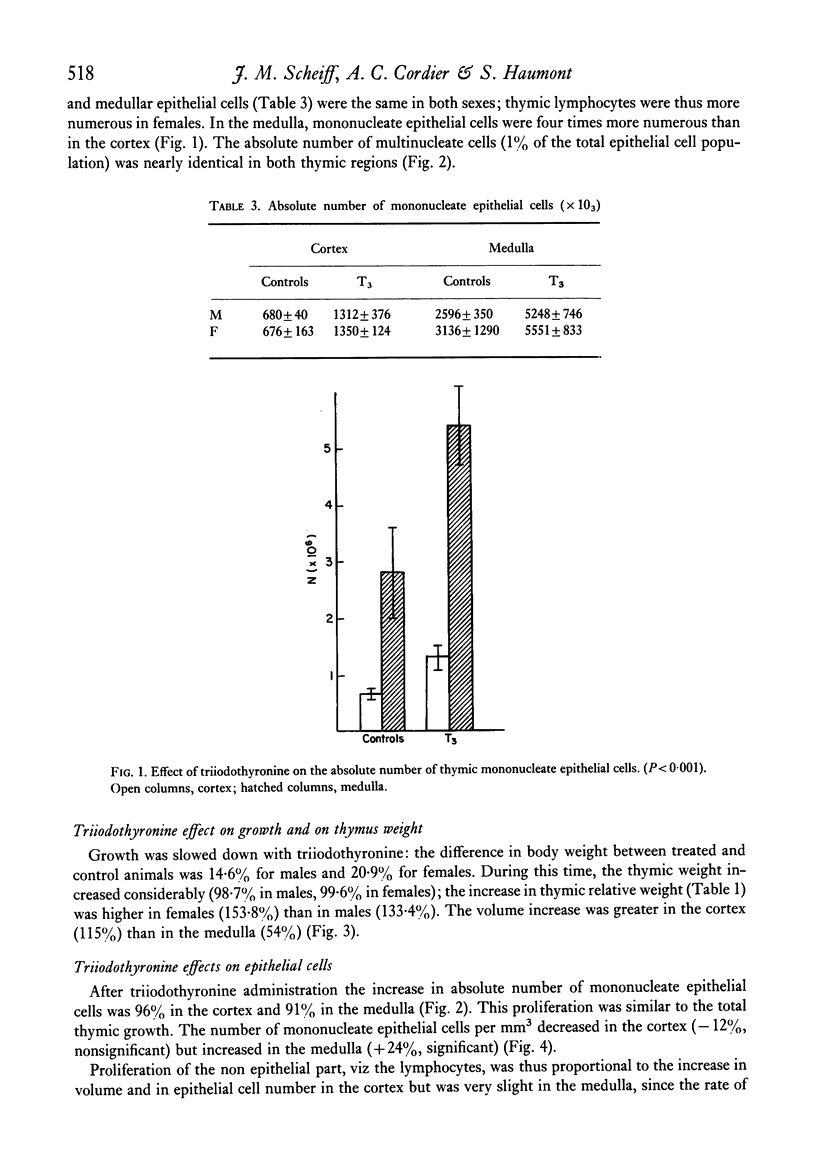
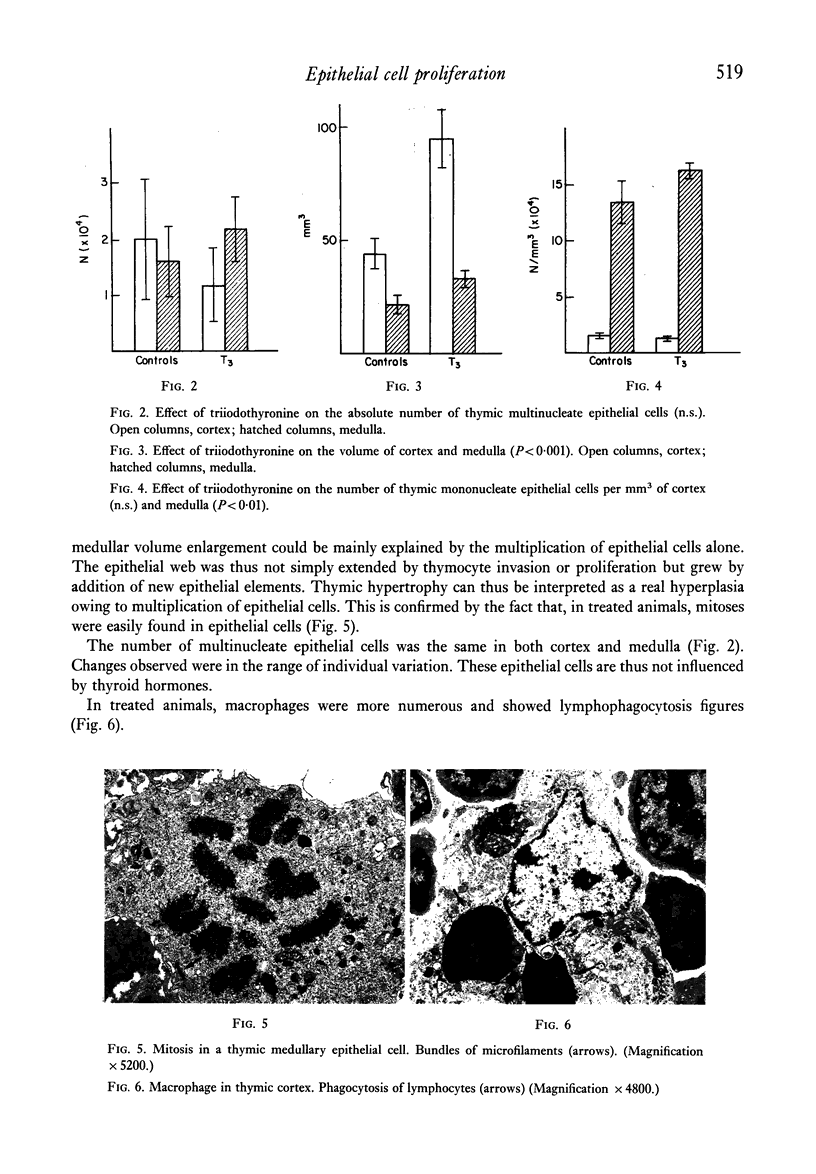


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dardenne M., Papiernik M., Bach J. F., Stutman O. Studies on thymus products. 3. Epithelial origin of the serum thymic factor. Immunology. 1974 Aug;27(2):299–304. [PMC free article] [PubMed] [Google Scholar]
- Ernström U., Larsson B. Thyroxin-stimulated venous output of small lymphocytes from the thymus. Acta Pathol Microbiol Scand. 1965;65(2):203–214. doi: 10.1111/apm.1965.65.2.203. [DOI] [PubMed] [Google Scholar]
- GUNN A., MICHIE W., IRVINE W. J. THE THYMUS IN THYROID DISEASE. Lancet. 1964 Oct 10;2(7363):776–778. doi: 10.1016/s0140-6736(64)90558-6. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. The isolation of thymopoietin (thymin). Ann N Y Acad Sci. 1975 Feb 28;249:177–185. doi: 10.1111/j.1749-6632.1975.tb29067.x. [DOI] [PubMed] [Google Scholar]
- Holm A. C., Lemarchand-Béraud T., Scazziga B. R., Cuttelod S. Human lymphocyte binding and deiodination of thyroid hormones in relation to thyroid function. Acta Endocrinol (Copenh) 1975 Dec;80(4):642–656. doi: 10.1530/acta.0.0800642. [DOI] [PubMed] [Google Scholar]
- IRVINE W. J., SUMERLING M. D. RADIOLOGICAL ASSESSMENT OF THE THYMUS IN THYROID AND OTHER DISEASES. Lancet. 1965 May 8;1(7393):996–999. doi: 10.1016/s0140-6736(65)91221-3. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARDER S. N. The effect of thyroxine on the lymphoid-tissue mass of immature female mice. J Natl Cancer Inst. 1951 Jun;11(6):1153–1161. [PubMed] [Google Scholar]
- METCALF D. The thymic origin of the plasma lymphocytosis stimulating factor. Br J Cancer. 1956 Sep;10(3):442–457. doi: 10.1038/bjc.1956.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie W., Beck J. S., Mahaffy R. G., Honein E. F., Fowler G. B. Quantitative radiological and histological studies of the thymus in thyroid disease. Lancet. 1967 Apr 1;1(7492):691–695. doi: 10.1016/s0140-6736(67)92177-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiff J. M. Multinucleate cells in thymus of C3H mice. Cell Tissue Res. 1976 Jul 30;170(3):305–314. doi: 10.1007/BF00219413. [DOI] [PubMed] [Google Scholar]
- Simpson J. G., Gray E. S., Michie W., Beck J. S. The influence of preoperative drug treatment on the extent of hyperplasia of the thymus in primary thyrotoxicosis. Clin Exp Immunol. 1975 Nov;22(2):249–255. [PMC free article] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- Tsai J. S., Samuels H. H. Thyroid hormone action: demonstration of putative nuclear receptors in human lymphocytes. J Clin Endocrinol Metab. 1974 May;38(5):919–922. doi: 10.1210/jcem-38-5-919. [DOI] [PubMed] [Google Scholar]
- Van Herle A. J., Chopra I. J. Thymic hyperplasia in Graves' disease. J Clin Endocrinol Metab. 1971 Feb;32(2):140–146. doi: 10.1210/jcem-32-2-140. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]