Abstract
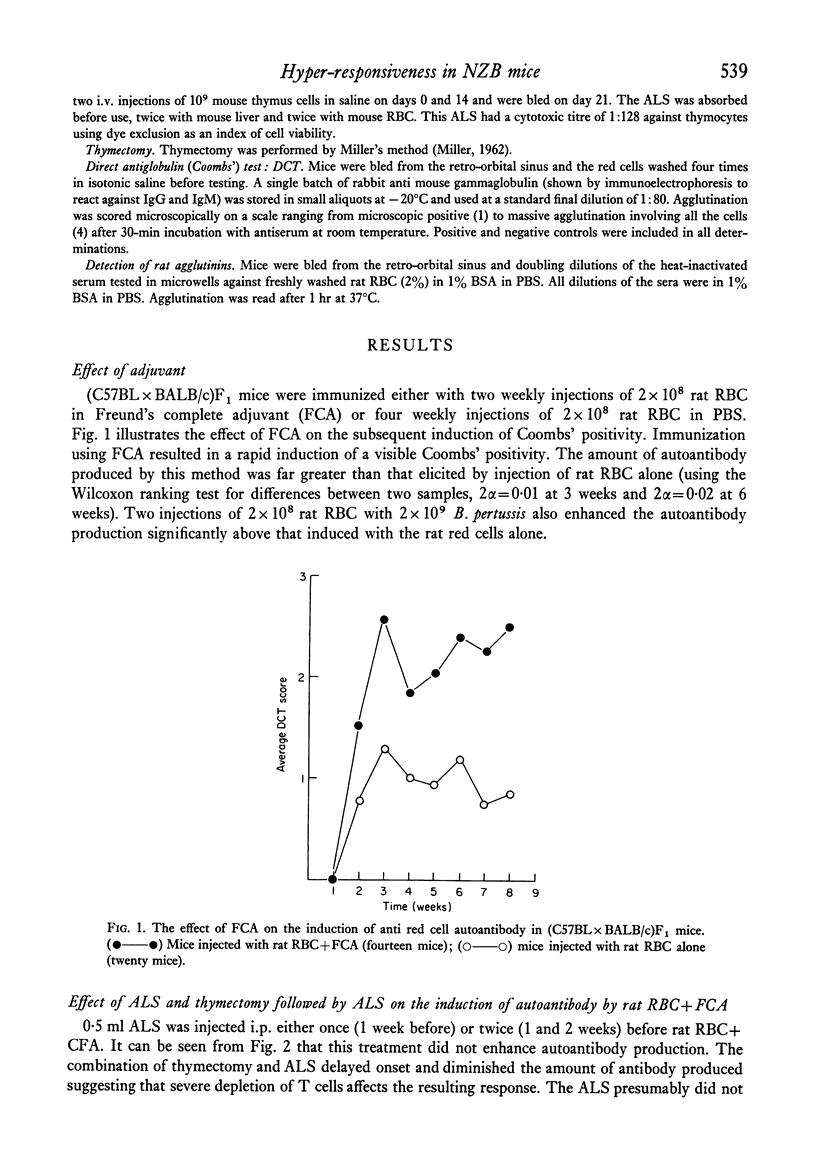
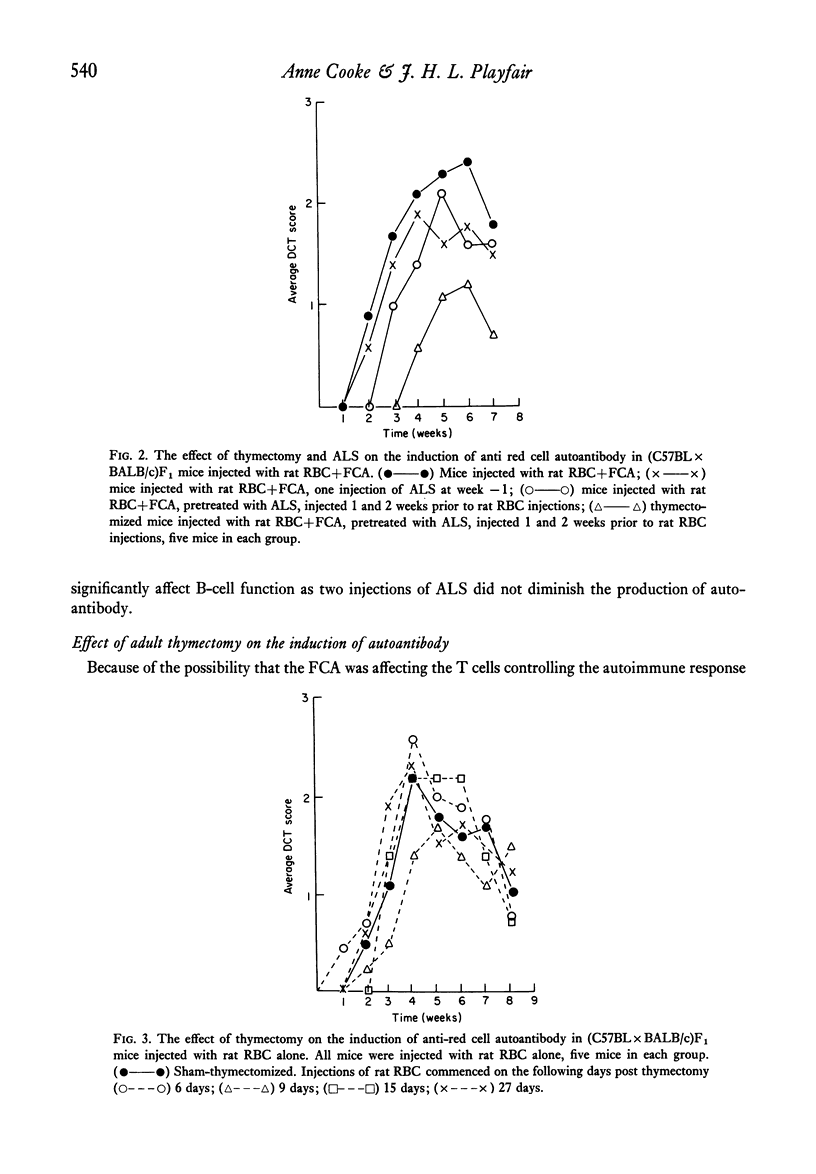
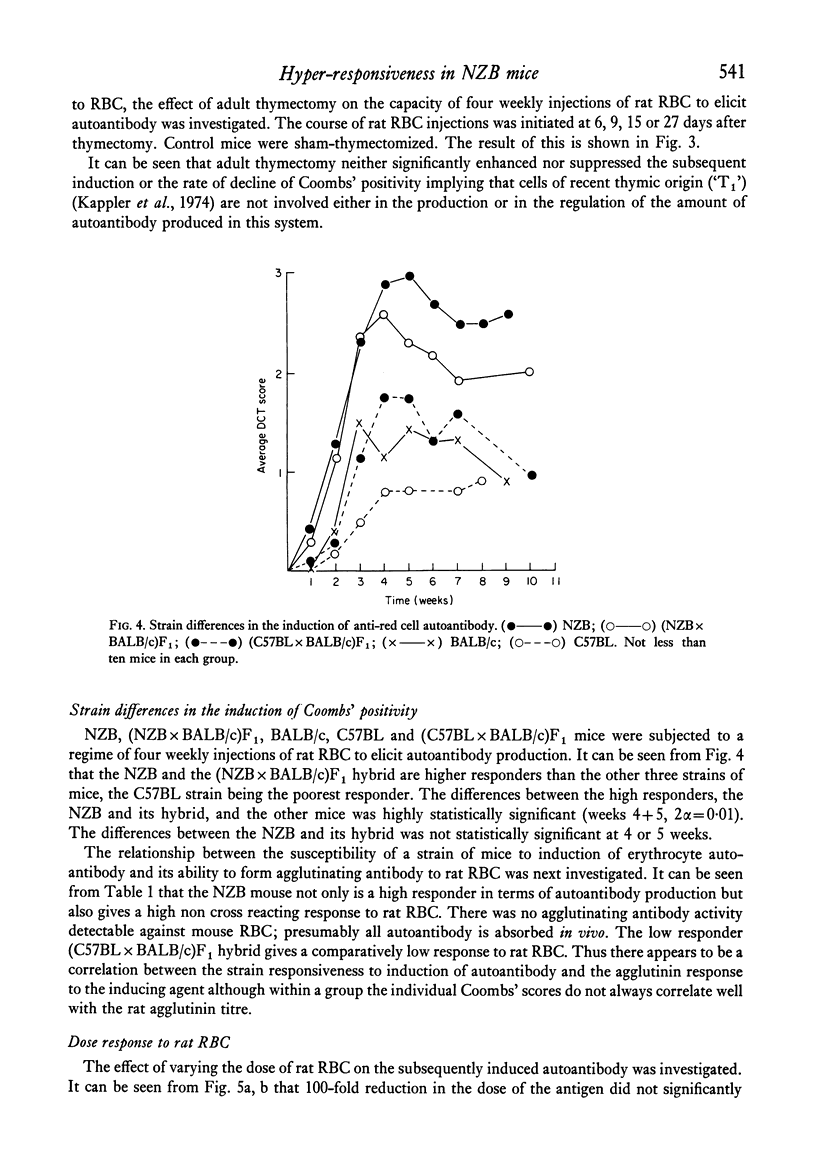
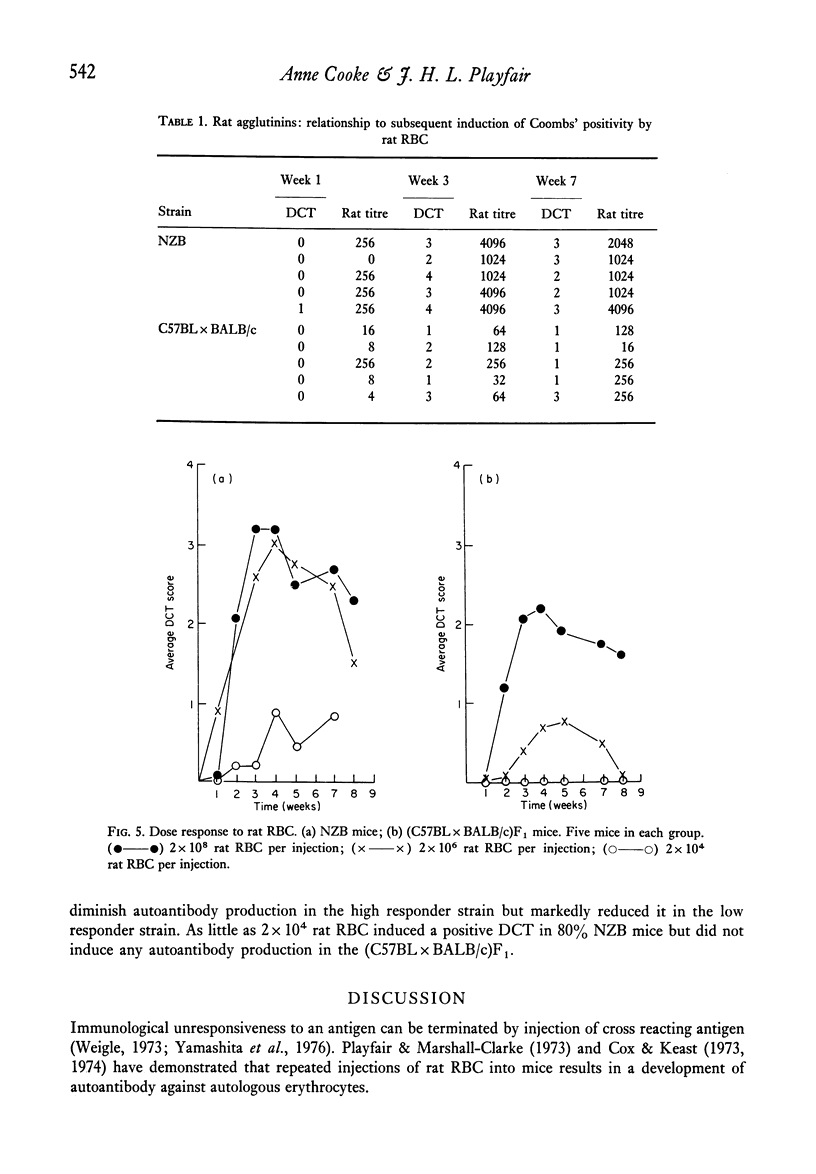
Strain differences in ease of induction of autoantibody production were observed when mice were injected with rat RBC. Responsiveness was not linked to the H-2 locus. NZB and (NZB X BALB/c)F1 mice were hyper-responsive both in terms of the induction of autoantibody and in the production of agglutinating antibody to rat RBC. C57BL/c and (C57BL X BALB/c)F1 were poor responders. Injection of the rat RBC in FCA converted a poor responder into a good responder. Adult thymectomy and ALS treatment did not significantly enhance autoantibody production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Aoki T., Boyse E. A., Old L. J., De Harven E., Hämmerling U., Wood H. A. G (Gross) and H-2 cell-surface antigens: location on Gross leukemia cells by electron microscopy with visually labeled antibody. Proc Natl Acad Sci U S A. 1970 Mar;65(3):569–576. doi: 10.1073/pnas.65.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEHME D. RESPONSE OF RETICULOENDOTHELIAL SYSTEM TO EXPERIMENTAL ALLERGIC ORCHITIS IN A GENETICALLY SUSCEPTIBLE AND RESISTANT MOUSE GENOTYPE. Proc Soc Exp Biol Med. 1965 Feb;118:374–376. doi: 10.3181/00379727-118-29847. [DOI] [PubMed] [Google Scholar]
- Chused T. M., Steinberg A. D., Parker L. M. Enhanced antibody response of mice to polyinosinic-polycytidylic acid by antithymocyte serum and its age-dependent loss in NZB-W mice. J Immunol. 1973 Jul;111(1):52–57. [PubMed] [Google Scholar]
- Cox K. O., Keast D. Autoimmune haemolytic anaemia induced in mice immunized with rat erythrocytes. Clin Exp Immunol. 1974 Jun;17(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Cox K. O., Keast D. Erythrocyte autoantibodies induced in mice immunized with rat erythrocytes. Immunology. 1973 Sep;25(3):531–539. [PMC free article] [PubMed] [Google Scholar]
- East J., De Sousa M. A., Prosser P. R., Jaquet H. Malignant changes in New Zealand black mice. Clin Exp Immunol. 1967 Jul;2(4):427–443. [PMC free article] [PubMed] [Google Scholar]
- East J., Harvey J. J., Tilly R. Transmission of auto-immune haemolytic anaemia and murine leukaemia virus in NZB-BALB/c hybrid mice. Clin Exp Immunol. 1976 Apr;24(1):196–209. [PMC free article] [PubMed] [Google Scholar]
- Gasser D. L., Newlin C. M., Palm J., Gonatas N. K. Genetic control of susceptibility to experimental allergic encephalomyelitis in rats. Science. 1973 Aug 31;181(4102):872–873. doi: 10.1126/science.181.4102.872. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Tursi A., Playfair J. H., Torrigiani G., Zamir R., Roitt I. M. Immunosuppressive potency and in-vitro activity of antilymphocyte globulin. Lancet. 1969 Jan 11;1(7585):68–72. doi: 10.1016/s0140-6736(69)91089-7. [DOI] [PubMed] [Google Scholar]
- HELYER B. J., HOWIE J. B. Spontaneous auto-immune disease in NZB/BL mice. Br J Haematol. 1963 Apr;9:119–131. doi: 10.1111/j.1365-2141.1963.tb05450.x. [DOI] [PubMed] [Google Scholar]
- Hughes R. A., Stedronska J. The susceptibility of rat strains to experimental allergic encephalomyelitis. Immunology. 1973 May;24(5):879–884. [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Hunter P. C., Jacobs D., Lord E. Functional heterogeneity among the T-derived lymphocytes of the mouse. I. Analysis by adult thymectomy. J Immunol. 1974 Jul;113(1):27–38. [PubMed] [Google Scholar]
- LEE J. M., OLITSKY P. K., SCHNEIDER H. A., ZINDER N. D. Role of heredity in experimental disseminated encephalomyelitis in mice. Proc Soc Exp Biol Med. 1954 Mar;85(3):430–432. doi: 10.3181/00379727-85-20906. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Jensen F., Kennel S. J., Dixon F. J., Des Roches G., Francke U. Karyotypic, virologic, and immunologic analyses of two continuous lymphocyte lines established from New Zealand black mice: possible relationship of chromosomal mosaicism to autoimmunity. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2965–2969. doi: 10.1073/pnas.69.10.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C., Aoki T., Huebner R. J. Further implication of murine leukemia-like virs in the disorders of NZB mice. J Exp Med. 1969 May 1;129(5):1045–1062. doi: 10.1084/jem.129.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C., Huang C. Y. Immunopathology of NZB/BL mice. V. Viruslike (filtrable) agent separable from lymphoma cells and identifiable by electron microscopy. J Exp Med. 1966 Dec 1;124(6):1031–1038. doi: 10.1084/jem.124.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Boyse E. A., de Harven E., Geering G. Group-specific viral antigens in the milk and tissues of mice naturally infected with mammary tumor virus or Gross leukemia virus. Virology. 1968 Apr;34(4):617–629. doi: 10.1016/0042-6822(68)90083-4. [DOI] [PubMed] [Google Scholar]
- Palmer D. W., Dauphinée M. J., Murphy E., Talal N. Hyperactive T-cell function in young NZB mice; increased proliferative responses to allogenic cells. Clin Exp Immunol. 1976 Mar;23(3):578–581. [PMC free article] [PubMed] [Google Scholar]
- Penhale W. J., Farmer A., Urbaniak S. J., Irvine W. J. Susceptibility of inbred rat strains to experimental thyroiditis: quantitation of thyroglobulin-binding cells and assessment of T-cell function in susceptible and non-susceptible strains. Clin Exp Immunol. 1975 Jan;19(1):179–191. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Marshall-Clarke S. Cross-reactions between erythrocytes at the T-cell level. Immunology. 1973 Mar;24(3):579–588. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Marshall-Clarke S. Induction of red cell autoantibodies in normal mice. Nat New Biol. 1973 Jun 13;243(128):213–214. doi: 10.1038/newbio243213a0. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune response of mice. I. The neonatal response to sheep red cells. Immunology. 1968 Jul;15(1):35–50. [PMC free article] [PubMed] [Google Scholar]
- Prosser P. R. Particles resembling murine leukaemia virus in New Zealand black mice. Clin Exp Immunol. 1968 Mar;3(3):213–226. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A., Allison A. C. A role of macrophages in the stimulation of immune responses by adjuvants. J Immunol. 1969 Jul;103(1):71–78. [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science. 1971 Dec 10;174(4014):1137–1139. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- Williams R. M., Moore M. J. Linkage of susceptibility to experimental allergic encephalomyelitis to the major histocompatibility locus in the rat. J Exp Med. 1973 Oct 1;138(4):775–783. doi: 10.1084/jem.138.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita U., Takami T., Hamaoka T., Kitagawa M. The role of hapten-reactive T lymphocytes in the induction of autoimmunity in mice. II. Termination of self-tolerance to erythrocytes by immunization with hapten-isologous erythrocytes. Cell Immunol. 1976 Jul;25(1):32–40. doi: 10.1016/0008-8749(76)90094-0. [DOI] [PubMed] [Google Scholar]


