Abstract
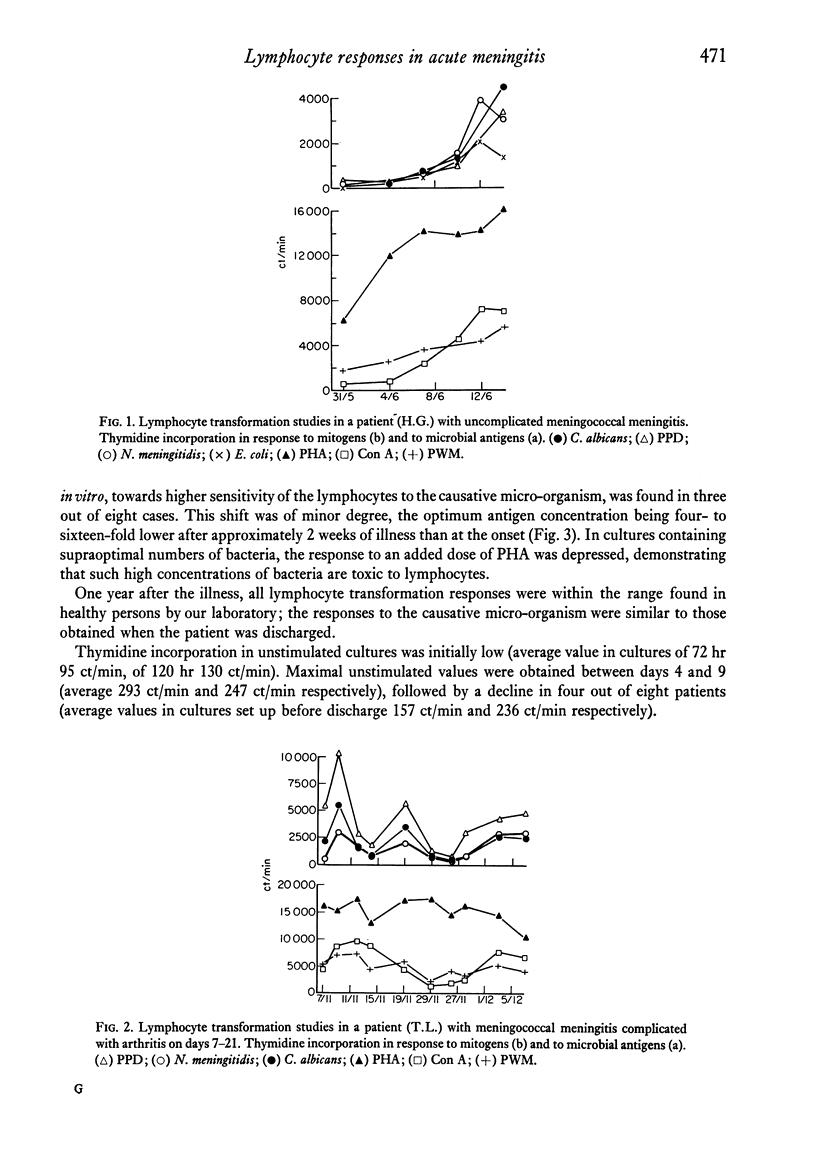
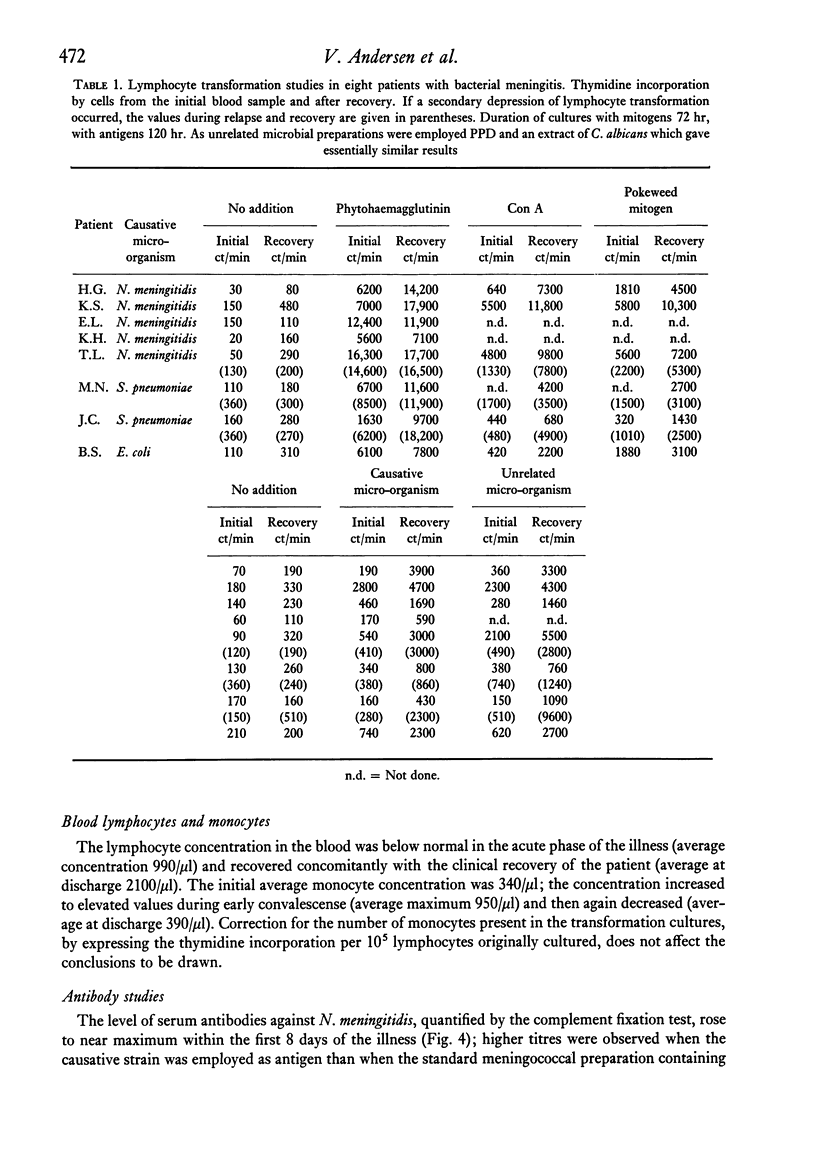
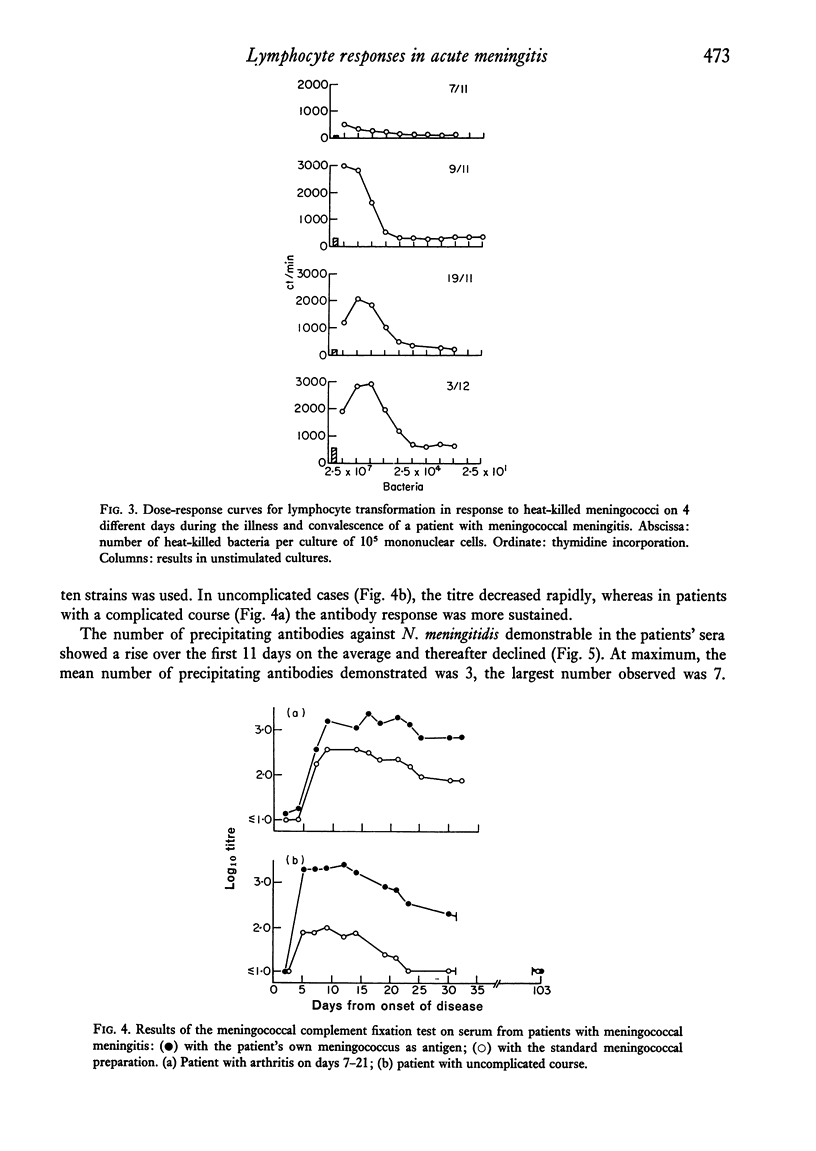
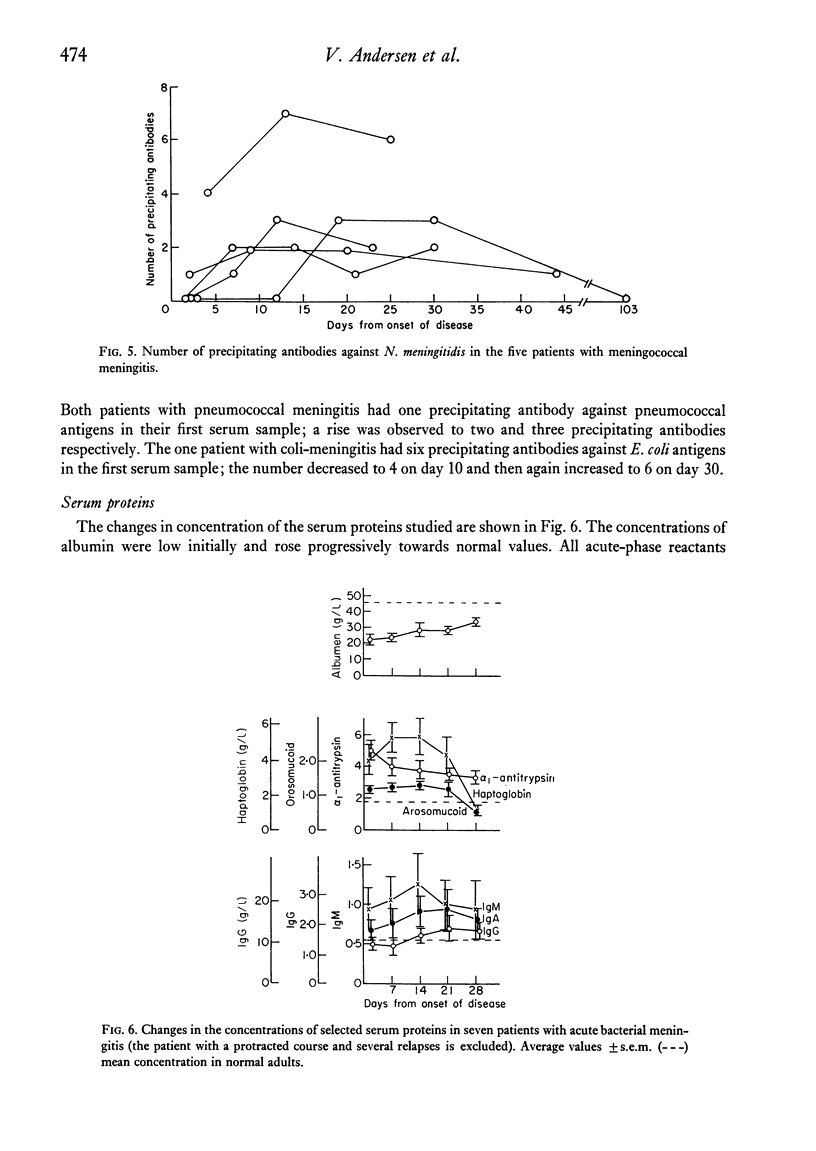
Lymphocyte transformation responses in vitro were studied in eight patients with acute bacterial meningitis (in five due to Neisseria meningitidis). Sequential studies were done from 24--48 hr after the first symptoms of infection to complete recovery. In all cases lymphocyte transformation was depressed during the acute phases of illness. The responses to microbial antigens were more affected than the responses to mitogens. The course of the lymphocyte responses to the causative micro-organism showed no difference from the responses to other microbial species. A moderate shift towards increased sensitivity of the lymphocytes to lower doses of the causative micro-organism was observed during the course of illness in three cases. In N. meningitidis infection, a rapid rise was seen in the serum titres of complement-fixing antibodies and in the number of precipitating antibodies, whereas the rise in immunoglobulin concentrations was more prolonged. Characteristic patterns of elevation and return towards normal were found in the serum concentrations of the acute-phase reactants alpha1-antitrypsin, haptoglobin, and orosomucoid. It is concluded that the lymphocyte transformation responses in vitro during severe bacterial infection are largely governed by non-specific factors, and that studies of lymphocyte responses to micro-organisms should always include other microbial species as controls.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiuti F., Ciarla M. V., D'Asero C., D'Amelio R., Garofalo J. A. Surface markers on lymphocytes of patients with infectious diseases. Infect Immun. 1973 Jul;8(1):110–117. doi: 10.1128/iai.8.1.110-117.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Hoffman T. A., Edwards E. A. Group-specific polysaccharide antigen and humoral antibody response in disease due to Neisseria meningitidis. J Infect Dis. 1972 Dec;126(6):636–644. doi: 10.1093/infdis/126.6.636. [DOI] [PubMed] [Google Scholar]
- Hoffmann G. W. A theory of regulation and self-nonself discrimination in an immune network. Eur J Immunol. 1975 Sep;5(9):638–647. doi: 10.1002/eji.1830050912. [DOI] [PubMed] [Google Scholar]
- Høiby N., Wiik A. Antibacterial precipitins and autoantibodies in serum of patients with cystic fibrosis. Scand J Respir Dis. 1975 May;56(1):38–46. [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- Niklasson P. M., Williams R. C., Jr Studies of peripheral blood T-and B-lymphocytes in acute infections. Infect Immun. 1974 Jan;9(1):1–7. doi: 10.1128/iai.9.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J. Modulation of in vitro lymphocyte transformation by antibodies: enhancement by antigen-antibody complexes and inhibition by antibody excess. Cell Immunol. 1972 Mar;3(3):341–360. doi: 10.1016/0008-8749(72)90243-2. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Shands J. W., Jr, Adler W. H., Smith R. T. Mitogenicity of bacterial endotoxins: characterization of the mitogenic principle. J Immunol. 1973 Aug;111(2):352–357. [PubMed] [Google Scholar]
- REYN A. LABORATORY DIAGNOSIS OF GONOCOCCAL INFECTIONS. Bull World Health Organ. 1965;32:449–469. [PMC free article] [PubMed] [Google Scholar]
- Rubin B., Wigzell H. Lymphocyte proliferation in vitro induced by hapten autologous protein conjugates. I. A study on the class of lymphocytes responding in vitro and on the nature and specificity of their receptors. J Exp Med. 1974 Mar 1;139(3):732–753. doi: 10.1084/jem.139.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Taranta A. Lymphocyte mitogens of staphylococcal origin. Ann N Y Acad Sci. 1974 Jul 31;236(0):362–375. doi: 10.1111/j.1749-6632.1974.tb41503.x. [DOI] [PubMed] [Google Scholar]
- Waller M. V., Miller G. V., Jr, Kelly J. J., 3rd Elevated titers of serum agglutinators. A serologic indicator of infection. Am J Clin Pathol. 1975 Jan;63(1):98–105. doi: 10.1093/ajcp/63.3.98. [DOI] [PubMed] [Google Scholar]


