Abstract
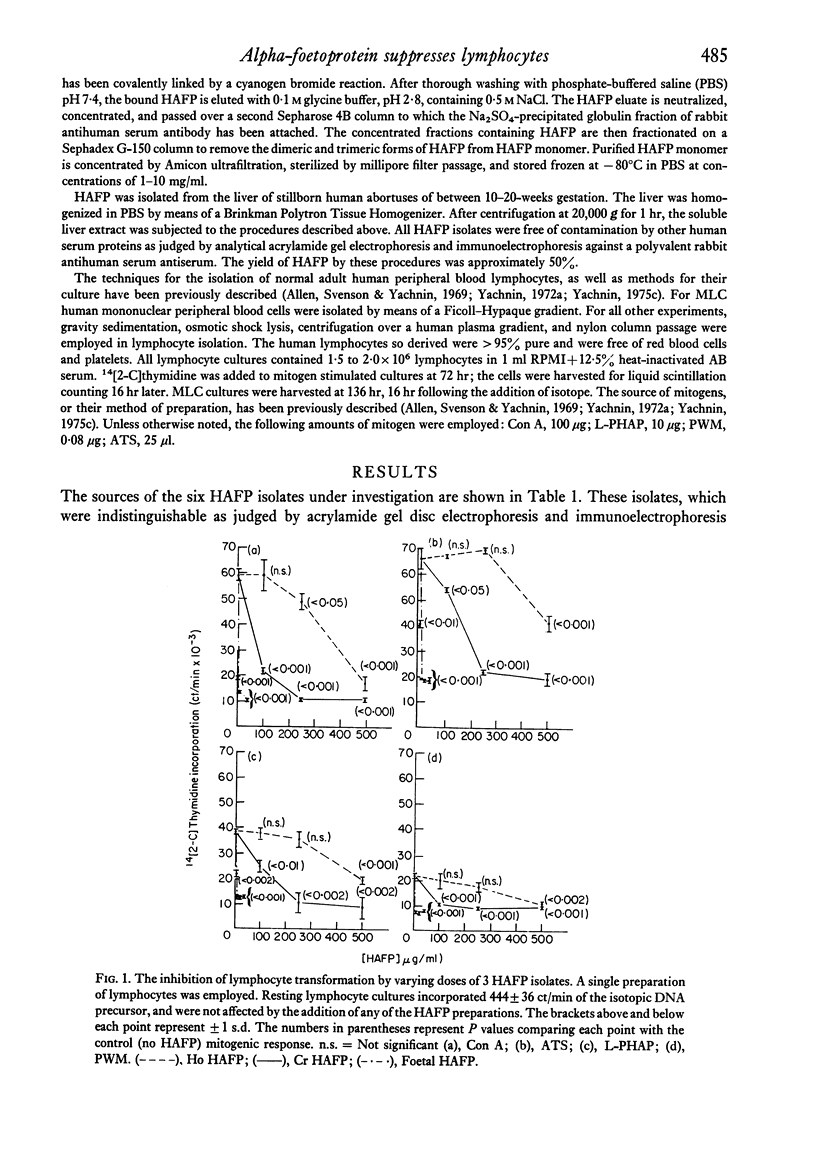
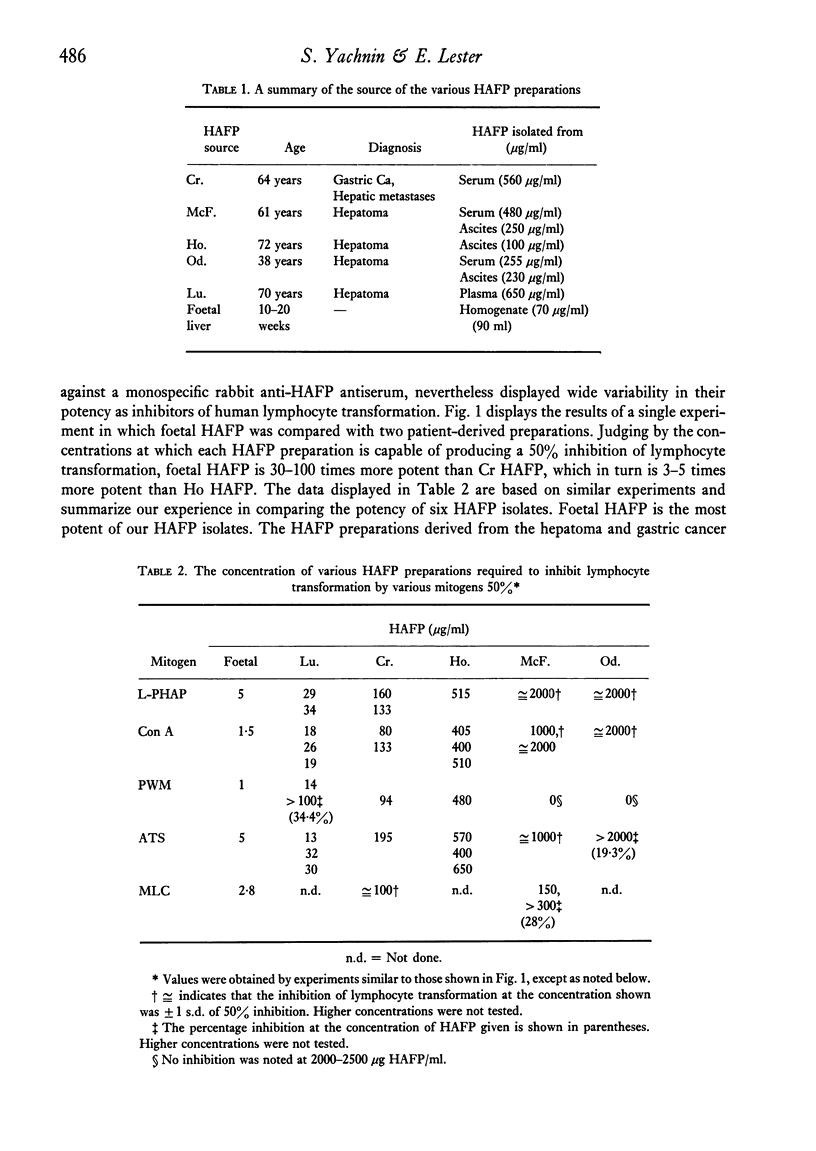
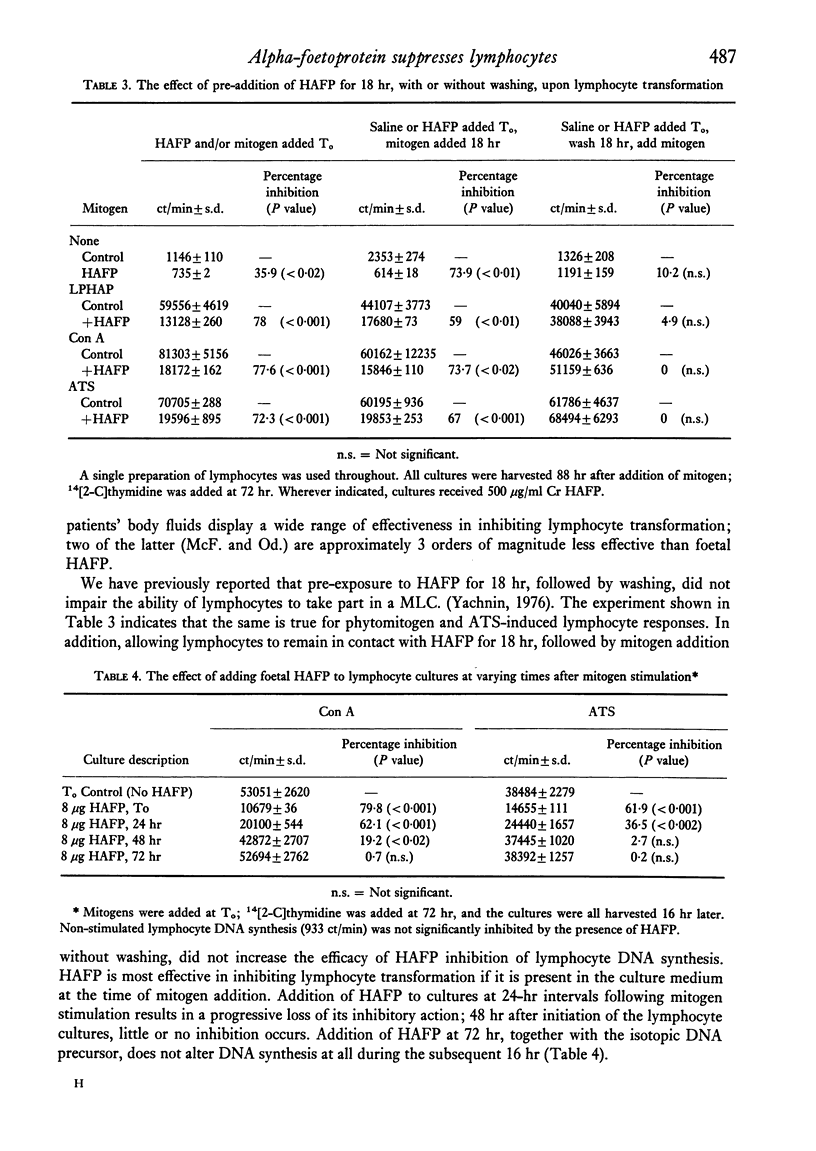
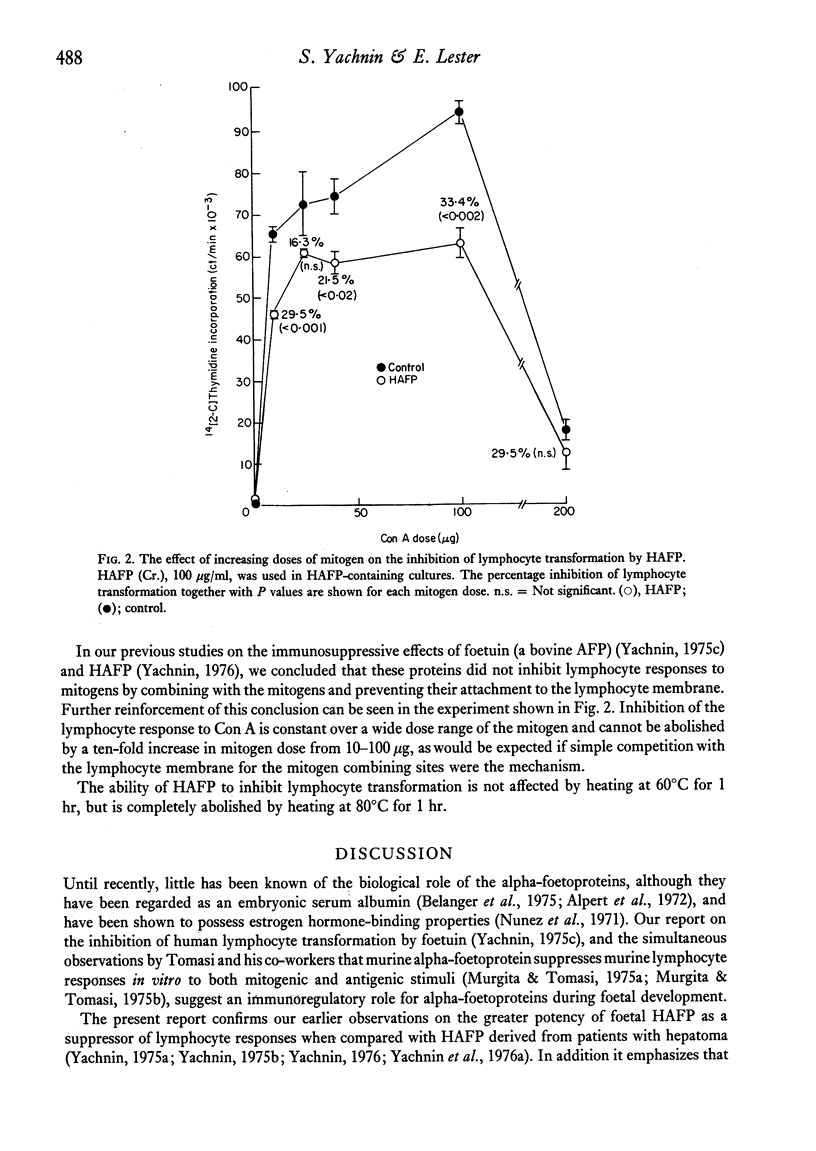
Five pure isolates of human alpha-foetoprotein (HAFP) from adults with tumours of the li er or stomach, as well as HAFP isolated from foetal liver, inhibit in vitro human lymphocyte transformation induced by phytomitogens, anti-human thymocyte serum, and the mixed lymphocyte culture. Foetal HAFP produces 50% inhibition at concentrations of 1-5 mug/ml. The HAFPs isolated from tumour-bearing adults are 1-3 orders of magnitude less potent (50% inhibition achieved at approximately 20, 130, 500, and 2000 mug/ml, respectively). In order to achieve maximum inhibition HAFP must be present at the time of mitogen addition; pre-exposure of lymphocytes to HAFP, followed by washing, does not result in lymphocyte suppression. The inhibiting effect of HAFP cannot be overcome by a ten-fold increase in mitogen concentration implying that HAFP does not act by simple competition with the lymphocyte membrane for the mitogen combining site. HAFP may play an immunoregulatory role during foetal development.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adcock E. W., 3rd, Teasdale T., August C. S., Cox S., Meschia G., Ballaglia T. C., Naughton M. A. Human chorionic gonadotropin: its possible role in maternal lymphocyte suppression. Science. 1973 Aug 31;181(4102):845–847. doi: 10.1126/science.181.4102.845. [DOI] [PubMed] [Google Scholar]
- Allen L. W., Svenson R. H., Yachnin S. Purification of mitogenic proteins derived from Phaseolus vulgaris: isolation of potent and weak phytohemagglutinins possessing mitogenic activity. Proc Natl Acad Sci U S A. 1969 Jun;63(2):334–341. doi: 10.1073/pnas.63.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert E., Drysdale J. W., Isselbacher K. J., Schur P. H. Human -fetoprotein. Isolation, characterization, and demonstration of microheterogeneity. J Biol Chem. 1972 Jun 25;247(12):3792–3798. [PubMed] [Google Scholar]
- Cooperband S. R., Badger A. M., Davis R. C., Schmid K., Mannick J. A. The effect of immunoregulatory globulin (IRA) upon lymphocytes in vitro. J Immunol. 1972 Jul;109(1):154–163. [PubMed] [Google Scholar]
- Dattwyler R. J., Murgita R. A., Tomasi T. B., Jr Binding of alpha-foetoprotein to murine T cells. Nature. 1975 Aug 21;256(5519):656–657. doi: 10.1038/256656a0. [DOI] [PubMed] [Google Scholar]
- Gustine D. L., Zimmerman E. F. Developmental changes in microheterogeneity of foetal plasma glycoproteins of mice. Biochem J. 1973 Mar;132(3):541–551. doi: 10.1042/bj1320541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgita R. A., Tomasi T. B., Jr Suppression of the immune response by alpha-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975 Feb 1;141(2):269–286. doi: 10.1084/jem.141.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E., Engelmann F., Benassayag C., Jayle M. F. Identification et purification préliminaire de la faeto-protéine liant les aestrogènes dans le sérum de rats nouveau-nés. C R Acad Sci Hebd Seances Acad Sci D. 1971 Aug 30;273(9):831–834. [PubMed] [Google Scholar]
- Yachnin S. Demonstration of the inhibitory effect of human alpha-fetoprotein on in vitro transformation of human lymphocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2857–2861. doi: 10.1073/pnas.73.8.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnin S. Fetuin, an inhibitor of lymphocyte transformation. The interaction of fetuin with phytomitogens and a possible role for fetuin in fetal development. J Exp Med. 1975 Jan 1;141(1):242–256. doi: 10.1084/jem.141.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnin S. Inhibition of phytohemagglutinin-induced lymphocyte transformation by globulins; lack of correlation with phytohemagglutinin precipitation by serum proteins. J Immunol. 1972 Mar;108(3):845–847. [PubMed] [Google Scholar]
- Yachnin S. The potentiation and inhibition by autologous red cells and platelets of human lymphocyte transformation induced by pokeweed mitogen concanavalin A, mercuric chloride, antigen, and mixed leucocyte culture. Clin Exp Immunol. 1972 May;11(1):109–124. [PMC free article] [PubMed] [Google Scholar]


