Abstract
Investigation of the nature of the increased erythrocyte-antibody (EA) binding activity of peripheral blood lymphocytes (PBL) from rheumatoid arthritis (RA) patients reported in the preceding paper has revealed that IgG is the active class of antibody in this rosette formation. Some IgM binding also occurs. SRBC sensitized with F(ab)2 preparations of IgG do not give rosette formation even at high concentrations. EA binding is inhibited by prior incubation of lymphocytes with heat-aggregated human IgG but antigen-antibody complexes did not give significant inhibition.
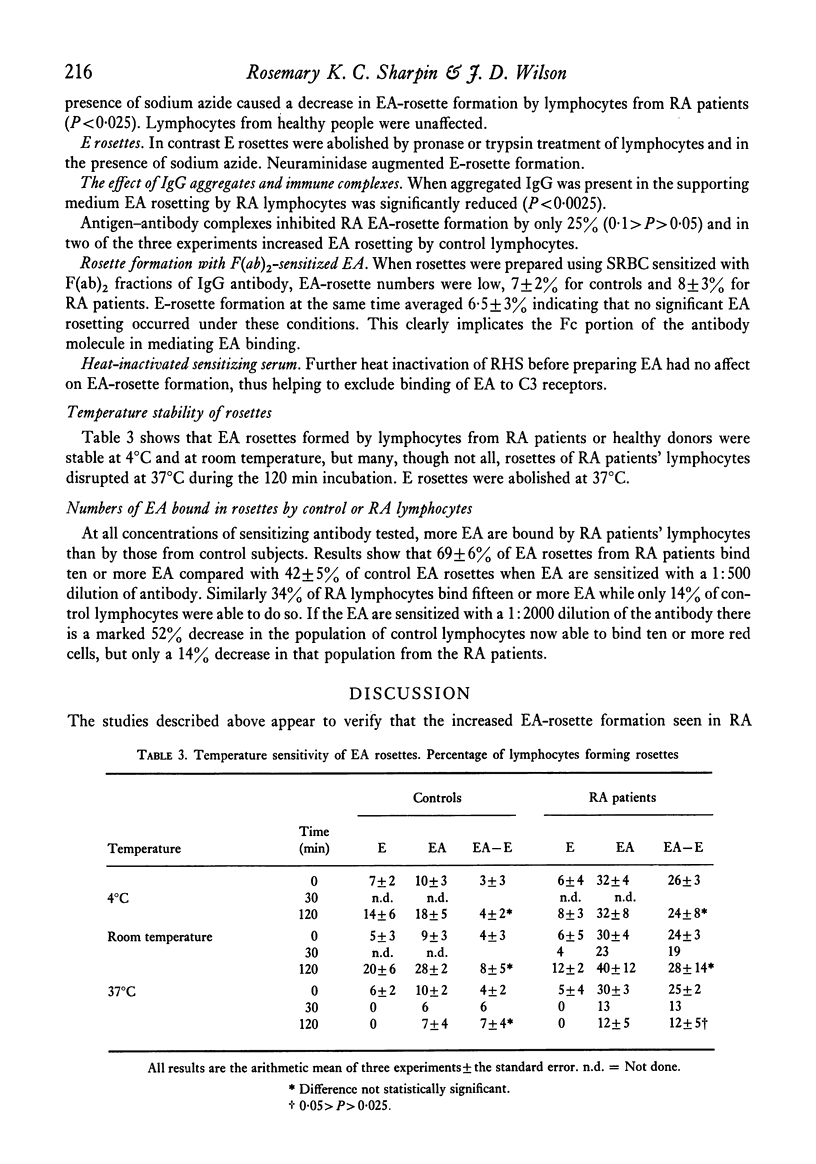
The majority of these rosettes were found to be stable at 4°C and room temperature but labile at 37°C.
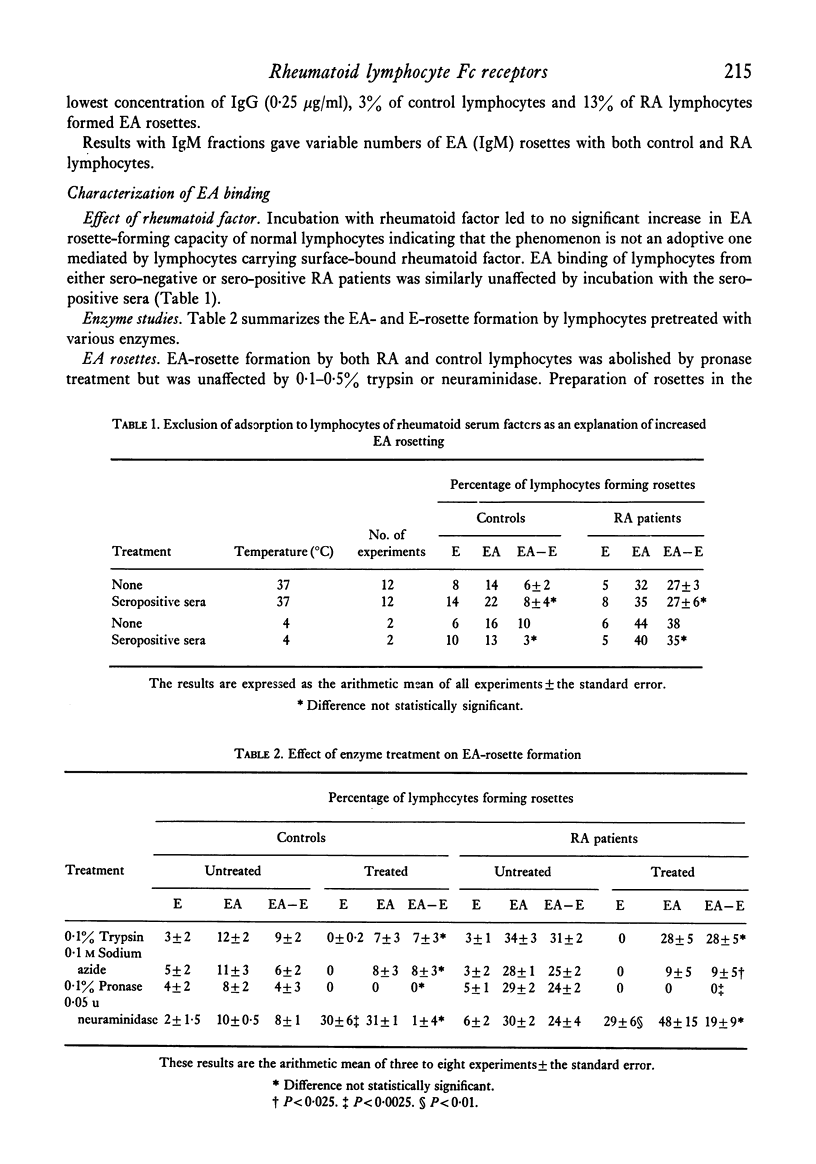
Enzyme studies with pronase, trypsin, neuraminidase and treatment with sodium azide gave results strongly supporting the conclusion that the increased binding observed is increased Fc-receptor activity. This activity appears not to be a result of Fc binding by cell-bound rheumatoid factor.
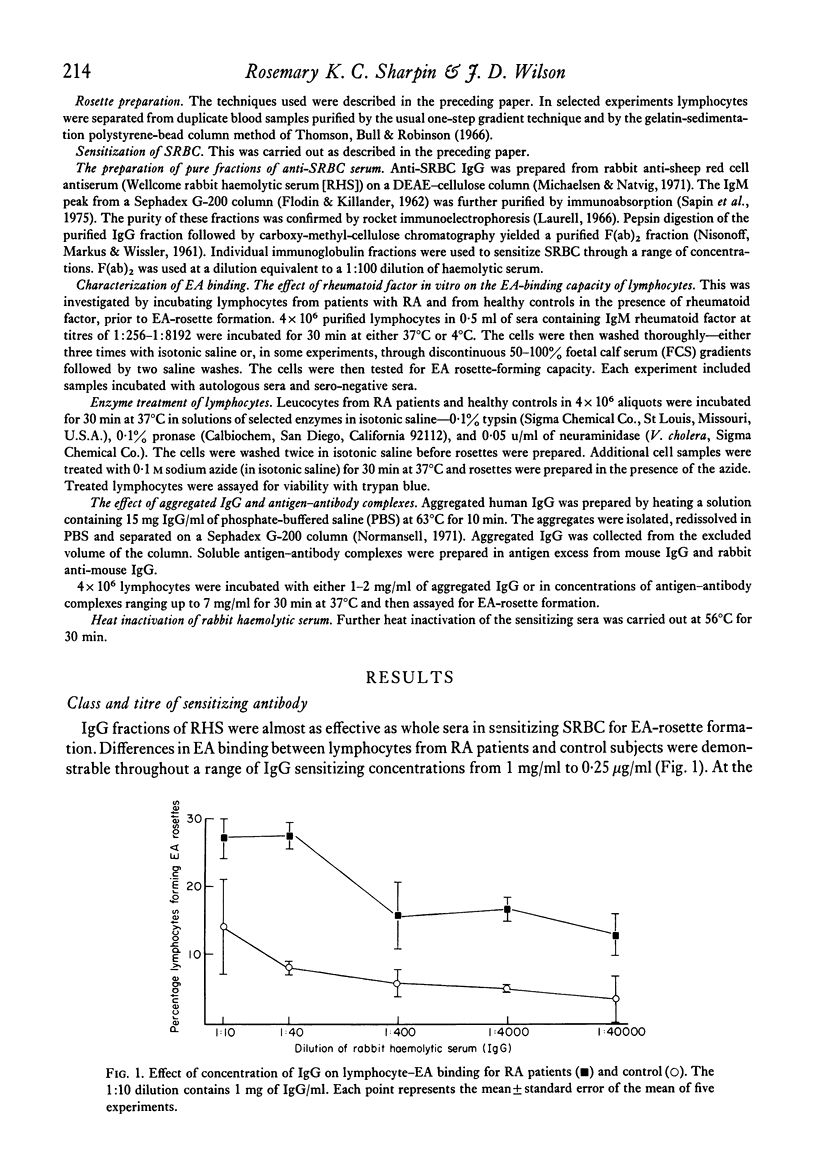
A range of titres of antibody and of IgG was used to sensitize erythrocytes to form EA and the enhanced EA-rosette formation by PBL from RA patients occurred throughout the range of concentrations of sensitizing antibody. Significantly more EA were bound by individual lymphocytes from RA patients than control subjects. This data suggest that the Fc receptors on RA lymphocytes are more avid for EA than receptors on lymphocytes from healthy people.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Warner N. L., Mandel T. A receptor for antibody on B lymphocytes. II. Immunochemical and electron microscopy characteristics. J Exp Med. 1972 Mar 1;135(3):627–642. doi: 10.1084/jem.135.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Skutelsky E., Kunkel H. G. Sheep red cell binding to human lymphocytes treated with neuraminidase; enhancement of T cell binding and identification of a subpopulation of B cells. J Exp Med. 1973 Jun 1;137(6):1532–1537. doi: 10.1084/jem.137.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B. Studies of the human lymphocyte receptor for heat-aggregated or antigen-complexed immunoglobulin. J Exp Med. 1974 Aug 1;140(2):508–522. doi: 10.1084/jem.140.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froland S. S., Natvig J. B. Identification of three different human lymphocyte populations by surface markers. Transplant Rev. 1973;16:114–162. doi: 10.1111/j.1600-065x.1973.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Froland S. S., Wisloff F., Michaelsen T. E. Human lymphocytes with receptors for IgG. A population of cells distinct from T- and B-lymphocytes. Int Arch Allergy Appl Immunol. 1974;47(1):124–138. doi: 10.1159/000231207. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Lobo P. I. Characterizaiton of two populations of human lymphocytes bearing easily detectable surface immunoglobulin. J Clin Invest. 1975 Dec;56(6):1464–1472. doi: 10.1172/JCI108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen T. E., Natvig J. B. Isolation and characterization of IgG subclass proteins and Fc fragments from normal human IgG. A method for utilizing 'non a' and 'non g' as genetic markers. Immunochemistry. 1971 Mar;8(3):235–242. doi: 10.1016/0019-2791(71)90477-0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., MARKUS G., WISSLER F. C. Separation of univalent fragments of rabbit antibody by reduction of a single, labile disulphide bond. Nature. 1961 Jan 28;189:293–295. doi: 10.1038/189293a0. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Hayward J. A. The lymphocyte surface. I. Relation between Fc receptors, C'3 receptors and surface immunoglobulin. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):47–63. doi: 10.1098/rspb.1974.0060. [DOI] [PubMed] [Google Scholar]
- Sapin C., Massez A., Contet A., Druet P. Isolation of normal human IgA, IgM and IgG fragments by polyacrylamide beads immunoadsorbents. J Immunol Methods. 1975 Nov;9(1):27–38. doi: 10.1016/0022-1759(75)90032-0. [DOI] [PubMed] [Google Scholar]
- Thomson A. E., Bull J. M., Robinson M. A. A procedure for separating viable lymphocytes from human blood and some studies on their susceptibility to hypotonic shocks. Br J Haematol. 1966 Jul;12(4):433–446. doi: 10.1111/j.1365-2141.1966.tb05652.x. [DOI] [PubMed] [Google Scholar]


