Abstract
Different affinity chromatography methods were compared for the isolation of C-reactive protein (CRP) from pathological fluids. In the presence of calcium ions CRP became bound to agar (Ganrot & Kindmark, 1969), to cyanogen bromide-activated agarose beads (Sepharose 4B) coupled with pneumococcal C substance (Osmand et al., 1975), to glutaraldehyde-activated polyacrylamide beads (Bio-Gel P300) or agarose-acrylamide beads (Ultrogel AcA 34) coupled with C substance and to `sulphated' polyacrylamide (Bio-Gel P300) beads. After thorough washing, elution with citrate or EDTA yielded CRP of varying degrees of purity. The use of agar provided high yields of heavily contaminated CRP which was substantially purified by passage through a column of insolubilized anti-normal human serum antibodies. Insolubilized C substance provided much purer CRP and the best yields were obtained from Sepharose–CNBr–C substance to which was attached considerably more ligand than was coupled to glutaraldehyde-activated beads. The only significant contaminant of the CRP isolated in this way was a normal serum protein, the P component of amyloid (Clt), which showed calcium-dependent binding to plain unsubstituted Sepharose and Ultrogel, as well as to polyacrylamide beads which had been treated with concentrated sulphuric acid. The use of these easily prepared `sulphated' polyacrylamide beads provides a method for isolating small quantities of CRP contaminated only by P component (Clt), without the need to first prepare C substance. Separation of CRP from P component was readily achieved by absorption of the P component onto agarose beads in the presence of calcium ions.
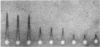
An `electroimmunodiffusion', `rocket' type of assay for pneumococcal C substance was developed, using CRP-rich fluid in the gel instead of antiserum.
Full text
PDF

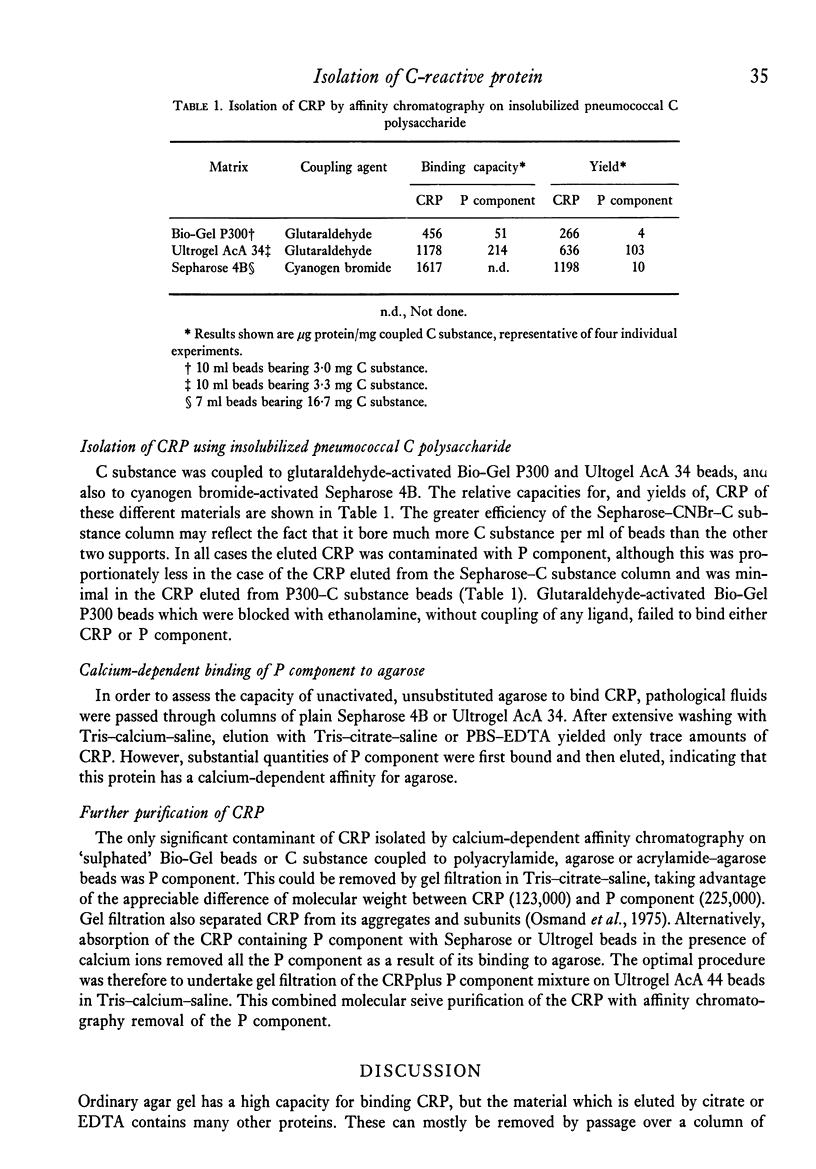


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimeh S. N., Painter R. H. The identification of a previously unrecognized subcomponent of the first component of complement. J Immunol. 1975 Aug;115(2):482–487. [PubMed] [Google Scholar]
- CATHCART E. S., COMERFORD F. R., COHEN A. S. IMMUNOLOGIC STUDIES ON A PROTEIN EXTRACTED FROM HUMAN SECONDARY AMYLOID. N Engl J Med. 1965 Jul 15;273:143–146. doi: 10.1056/NEJM196507152730306. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O., Kindmark C. O. A simple two-step procedure for isolation of C-reactive protein. Biochim Biophys Acta. 1969 Dec 23;194(2):443–448. doi: 10.1016/0005-2795(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y. Structural and immunological studies on the pneumococcal C polysaccharide. J Biol Chem. 1967 Feb 10;242(3):463–470. [PubMed] [Google Scholar]
- Kindmark C. O. Quantitative measurement of C-reactive protein in serum. Clin Chim Acta. 1969 Oct;26(1):95–98. doi: 10.1016/0009-8981(69)90291-5. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet. 1977 May 14;1(8020):1029–1031. doi: 10.1016/s0140-6736(77)91260-0. [DOI] [PubMed] [Google Scholar]
- Skinner M., Cohen A. S., Shirahama T., Cathcart E. S. P-component (pentagonal unit) of amyloid: isolation, characterization, and sequence analysis. J Lab Clin Med. 1974 Oct;84(4):604–614. [PubMed] [Google Scholar]