Abstract
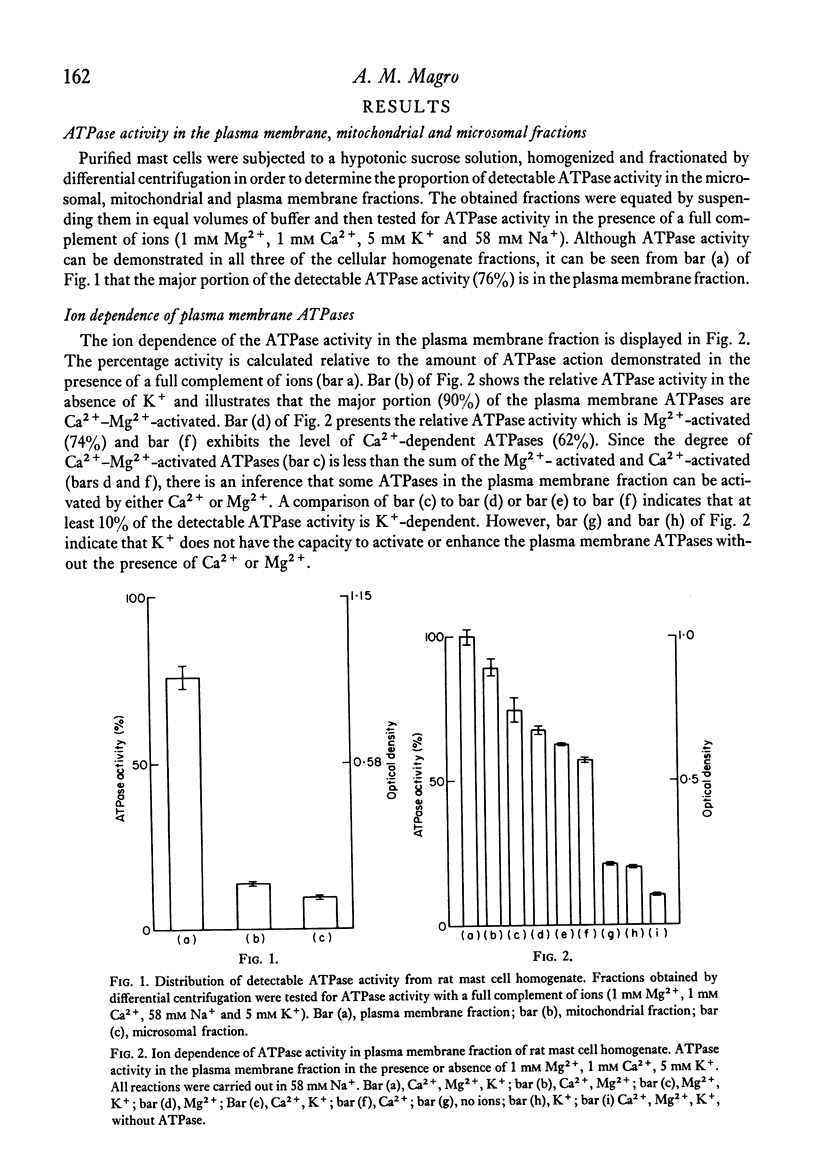
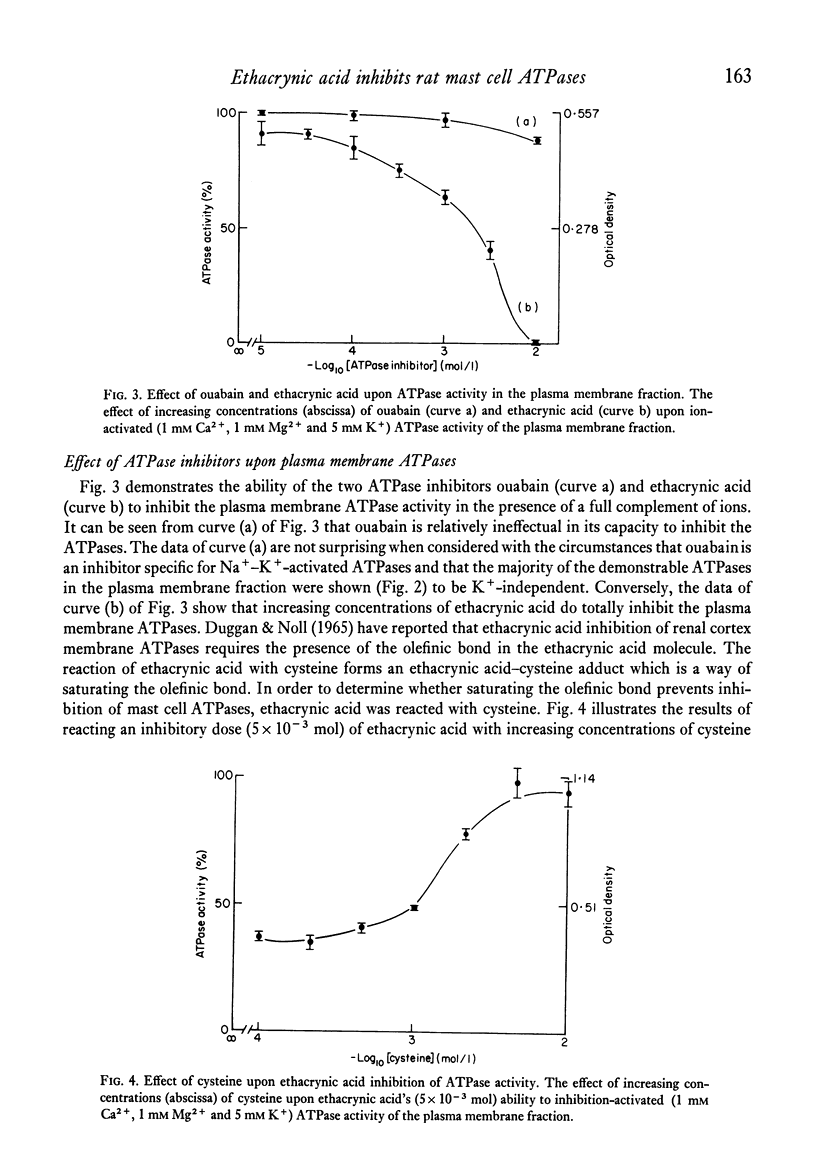
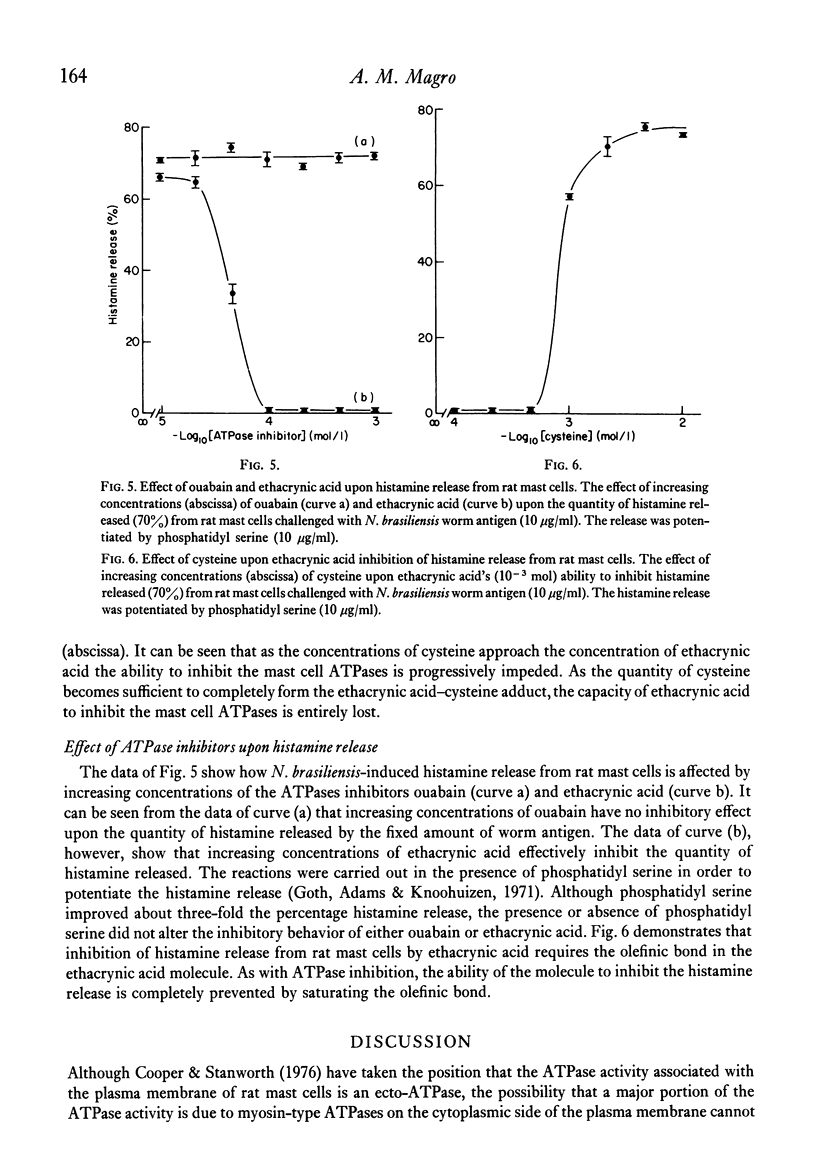
A crude plasma membrane fraction from the homogenate of purified rat mast cells demonstrates a high degree of Ca2+-dependent and Mg2+-dependent adenosine triphosphatase (ATPase) activity. The microsomal and mitochondrial fractions show negligible amounts of the Ca2+ and Mg2+-activated ATPases. The broad ATPase inhibitor, ethacrynic acid, effectively blocks the mast cell ATPase activity while ouabain demonstrates little inhibitory effect. Correspondingly, ethacrynic acid inhibits histamine release from antigen-challenged mast cells while ouabain does not. Both ATPase inhibition and histamine release inhibition by ethacrynic acid require the presence of the olefinic bond in the ethacrynic acid molecule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONTING S. L., CARAVAGGIO L. L., HAWKINS N. M. Studies on sodium-potassium-activated adenosinetriphosphatase. IV. Correlation with cation transport sensitive to cardiac glycosides. Arch Biochem Biophys. 1962 Sep;98:413–419. doi: 10.1016/0003-9861(62)90206-0. [DOI] [PubMed] [Google Scholar]
- BONTING S. L., SIMON K. A., HAWKINS N. M. Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative distribution in several tissues of the cat. Arch Biochem Biophys. 1961 Dec;95:416–423. doi: 10.1016/0003-9861(61)90170-9. [DOI] [PubMed] [Google Scholar]
- Boegman R. J., Manery J. F., Pinteric L. The separation and partial purification of membrane-bound (Na + + K + )-dependent Mg 2+ -ATPase and (Na + +K + (Na + +K + )-independent Mg 2+ -ATPase from frog skeletal muscle. Biochim Biophys Acta. 1970 Jun 2;203(3):506–530. doi: 10.1016/0005-2736(70)90189-6. [DOI] [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D., Koehler J. K. Electron microscopy of freeze-fractured rat peritoneal mast cells. J Ultrastruct Res. 1975 Apr;51(1):46–54. doi: 10.1016/s0022-5320(75)80007-4. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Stanworth D. R. Characterization of calcium-ion-activated adenosine triphosphatase in the plasma membrane of rat mast cells. Biochem J. 1976 Jun 15;156(3):691–700. doi: 10.1042/bj1560691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant B., Norn S., Felding P., Olsen N., Ziebell A., Nissen J. ATP level and CO2 production of mast cells in anaphylaxis. Int Arch Allergy Appl Immunol. 1974;47(6):894–908. doi: 10.1159/000231280. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. M., Gomperts B. D. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cells. Nature. 1977 Feb 17;265(5595):635–636. doi: 10.1038/265635a0. [DOI] [PubMed] [Google Scholar]
- Goth A., Adams H. R., Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971 Sep 10;173(4001):1034–1035. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- Johansen T., Chakravarty N. The utilization of adenosine triphosphate in rat mast cells during histamine release induced by anaphylactic reaction and compound 48/80. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(2-3):243–260. doi: 10.1007/BF00500530. [DOI] [PubMed] [Google Scholar]
- Kojima S., Yokogawa M., Tada T. Production and properties of reaginic antibodies in rabbits infected with Clonorchis sinensis or Schistosoma japonicum. Exp Parasitol. 1974 Feb;35(1):141–149. doi: 10.1016/0014-4894(74)90017-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro A. M. Blocking of histamine release from human basophils in vitro by the ATPase inhibitor, ethacrynic acid. Clin Exp Immunol. 1977 Sep;29(3):436–441. [PMC free article] [PubMed] [Google Scholar]
- Mongar J. L., Svec P., Foreman J. C. Aanphylactic secretion of histamine in relation to the interaction of phospholipids with divalent cations. Int Arch Allergy Appl Immunol. 1973;45(1):70–73. doi: 10.1159/000231004. [DOI] [PubMed] [Google Scholar]
- OGILVIE B. M. REAGIN-LIKE ANTIBODIES IN ANIMALS IMMUNE TO HELMINTH PARASITES. Nature. 1964 Oct 3;204:91–92. doi: 10.1038/204091a0. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M. Reagin-like antibodies in rats infected with the nematode parasite Nippostrongylus brasiliensis. Immunology. 1967 Feb;12(2):113–131. [PMC free article] [PubMed] [Google Scholar]
- Orr T. S., Hall D. E., Allison A. C. Role of contractile microfilaments in the release of histamine from mast cells. Nature. 1972 Apr 14;236(5346):350–351. doi: 10.1038/236350a0. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- Probst E., Lüscher F. Studies on thrombosthenin a, the actin-like moiety of the contractile protein from blood platelets. I. Isolation, characterization and evidence for two forms of thrombosthenin A. Biochim Biophys Acta. 1972 Oct 31;278(3):577–584. doi: 10.1016/0005-2795(72)90017-7. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. The difference in sensitivity to cardiac steroids of (Na++K+)-stimulated ATPase and amino acid transport in the intestinal mucosa of the rat and other species. J Physiol. 1970 Jan;206(1):41–60. doi: 10.1113/jphysiol.1970.sp008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Stenson W., Parker C. W. Modulation of cyclic AMP in purified rat mast cells. I. Responses to pharmacologic, metabolic, and physical stimuli. J Immunol. 1975 May;114(5):1473–1479. [PubMed] [Google Scholar]
- Tilney L. G., Mooseker M. Actin in the brush-border of epithelial cells of the chicken intestine. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2611–2615. doi: 10.1073/pnas.68.10.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Z., Perdue J. F. Contractile proteins of cultured cells. I. The isolation and characterization of an actin-like protein from cultured chick embryo fibroblasts. J Biol Chem. 1972 Jul 25;247(14):4503–4509. [PubMed] [Google Scholar]


