Abstract
The present study investigated the effect of dexamethasone (DEX) administration on different populations of mononuclear cells and neutrophils mediating antibody-dependent cellular cytotoxicity (ADCC) against different target cells.
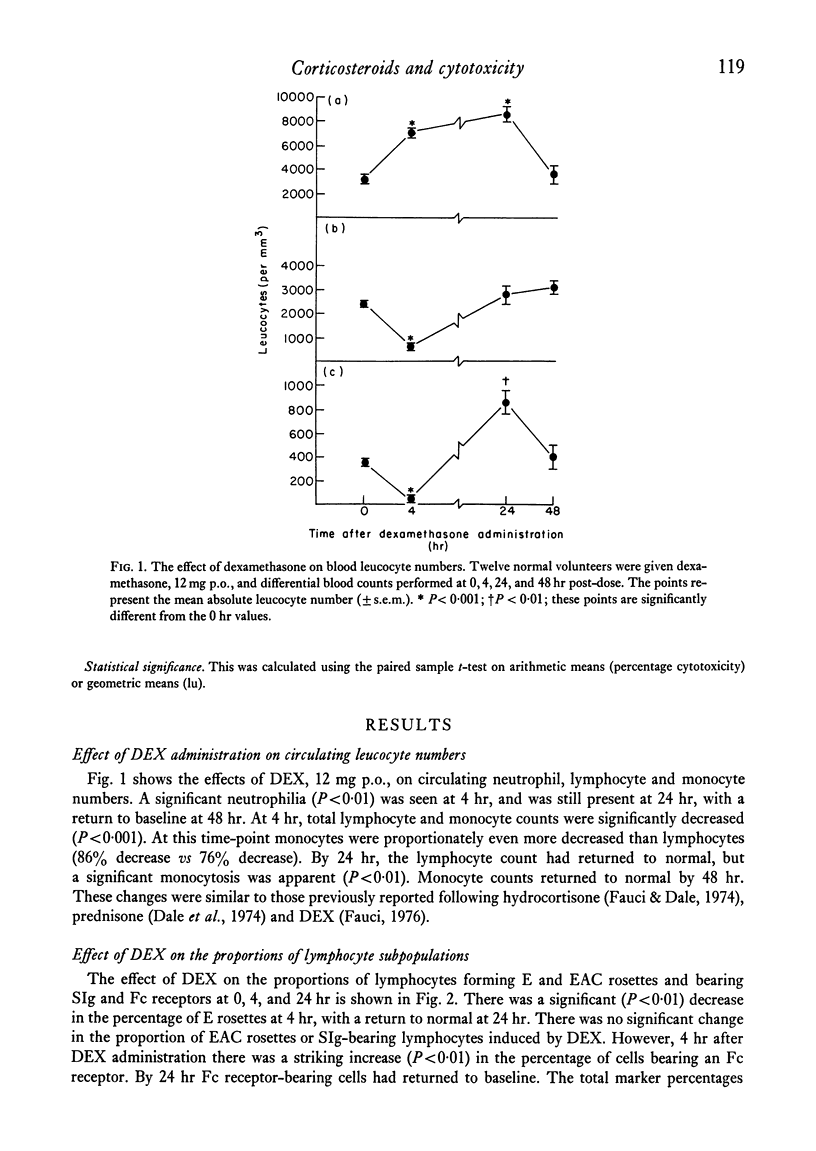
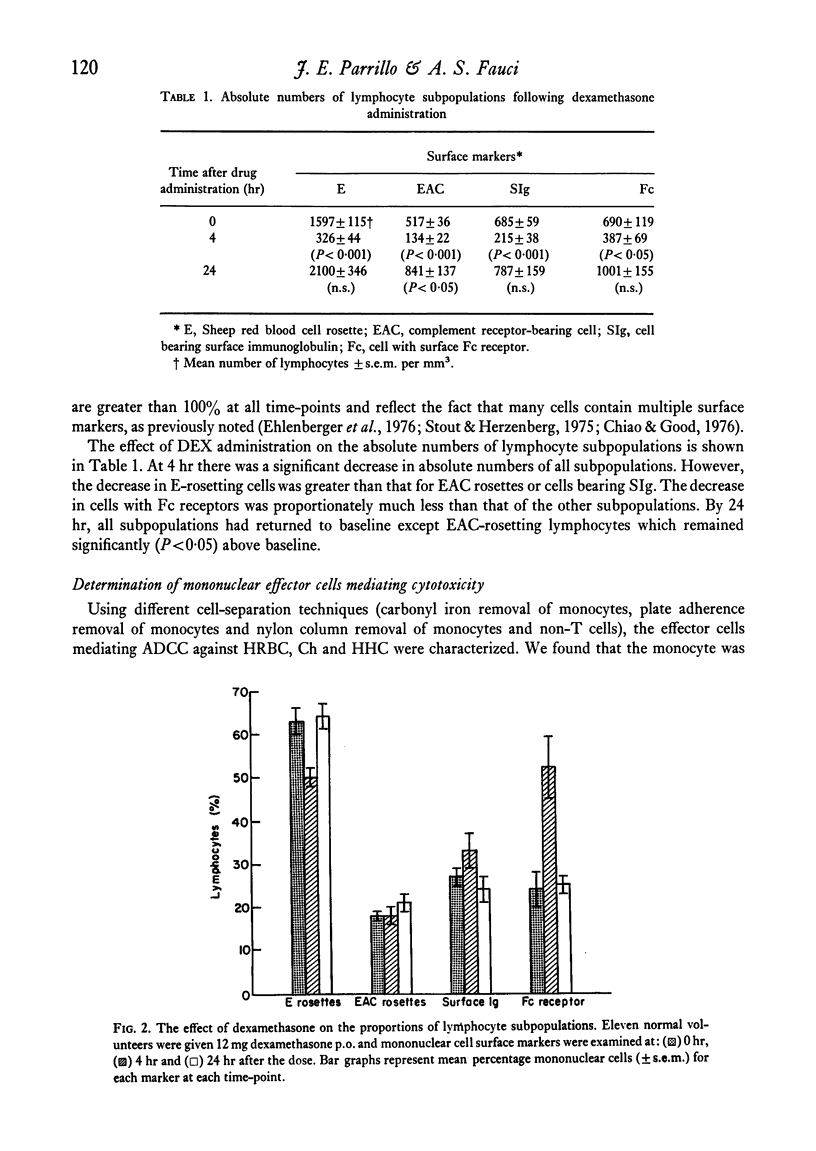
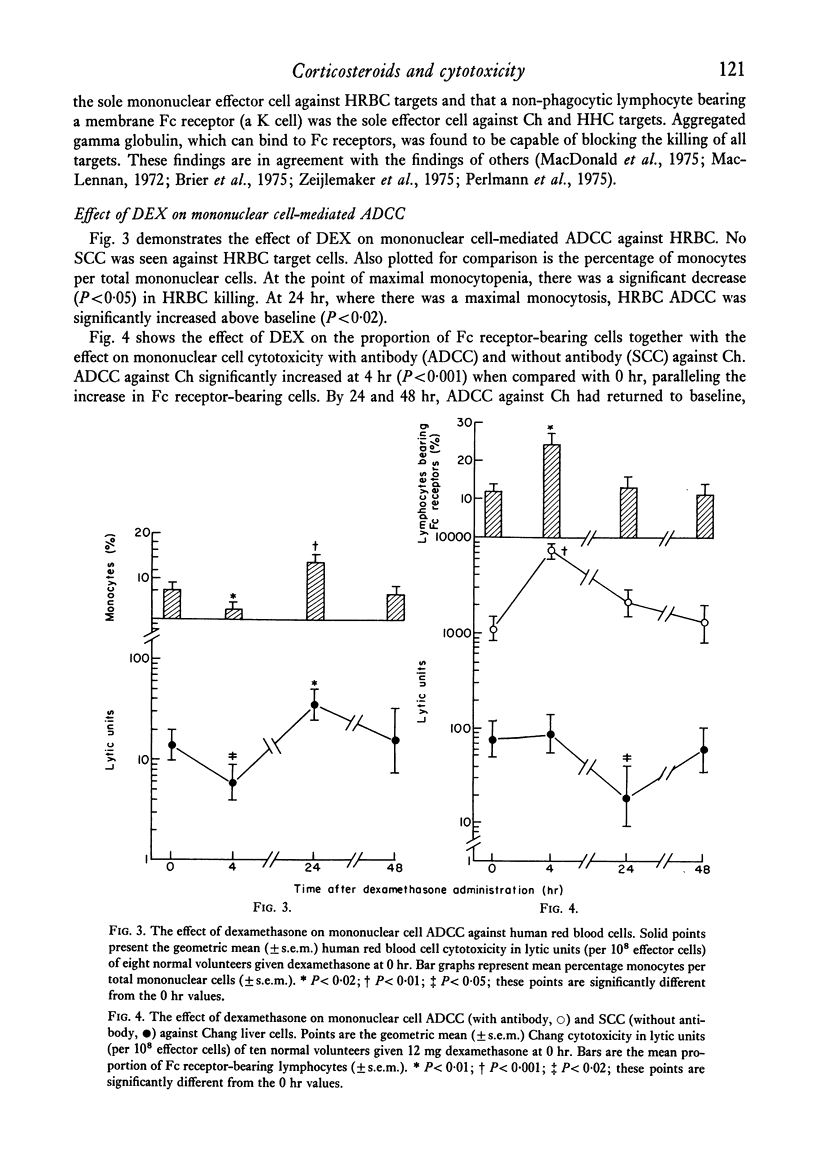
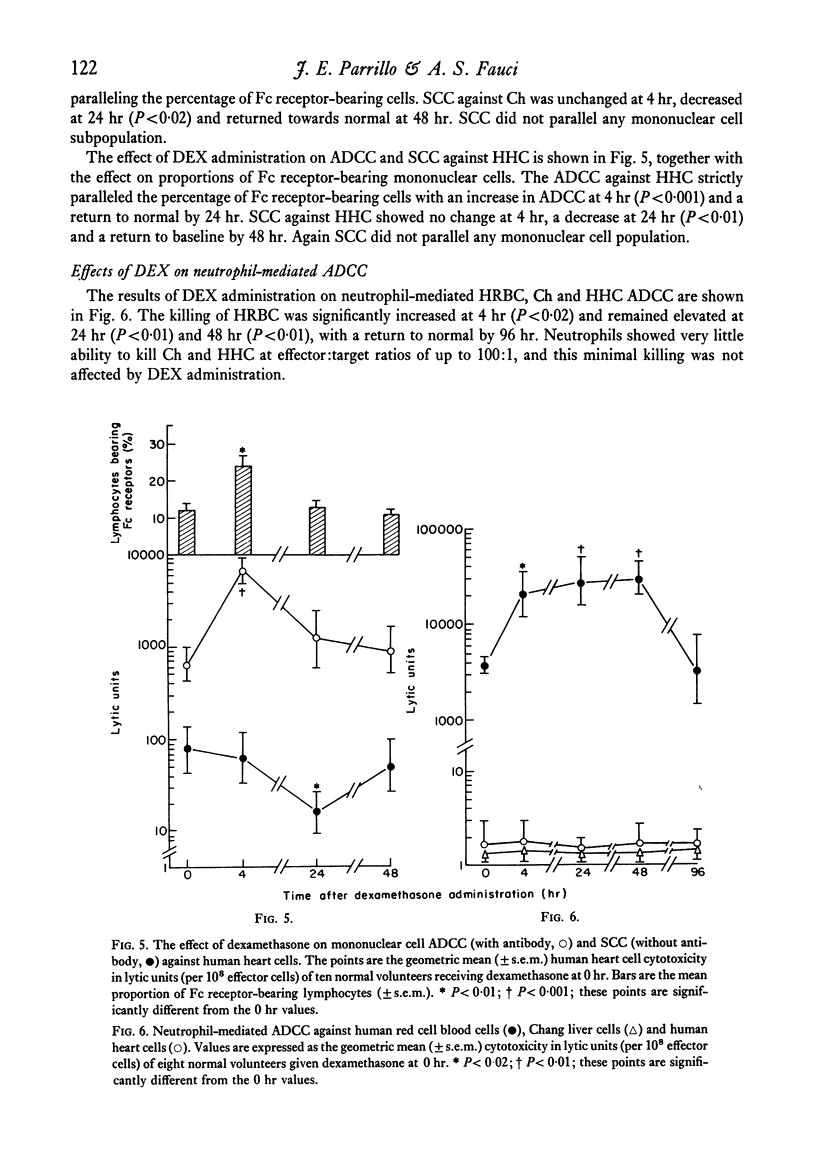
Mononuclear cells (lymphocytes and monocytes) and neutrophils were obtained from twenty-seven normal volunteers at 0, 4, 24 and 48 hr after oral administration of 21 mg of DEX. ADCC was determined utilizing the following targets: human red blood cells (HRBC), Chang liver cells (Ch) and human heart cells (HHC). The predominant mononuclear effector in HRBC killing was shown to be a monocyte and in Ch and HHC killing, a K cell. As previously shown, DEX produced a profound monocytopenia and lymphocytopenia at 4 hr with a return of lymphocyte counts to normal and monocyte counts to supra-normal at 24 hr. At the point of maximal monocytopenia, monocyte-mediated HRBC killing decreased from a geometric mean of 14 to 4 lytic units per 108 effector cells (P<0·05) and rebounded at 24 hr to a mean of 39 lytic units (P<0·02) with the rebound monocytosis. At the point of absolute lymphopenia (4 hr), there was a relative enrichment in the proportion of lymphocytes bearing an Fc receptor (K cells, P<0·01). Concomitant with this was an increase in ADCC against Ch and HHC from geometric means of 1121 to 7172 lytic units and 939 to 7354 lytic units (P<0·001) respectively. Thus, a major action of DEX administration on mononuclear ADCC was to differentially enrich or deplete different effector cells to and from the circulation, causing changes in cytotoxicity. Since the cytotoxicity paralleled the proportion of effector cells, the cells remaining in the circulation following DEX administration retained normal antibody-dependent cytotoxic capabilities.
Neutrophil-mediated ADCC against HRBC significantly increased at 4 hr from a geometric mean of 3785 to 20142 lytic units (P<0·02) concomitant with the blood neutrophilia and remained elevated for 72 hr. Neutrophil ADCC against Ch and HHC was minimal and was not affected by DEX administration.
This study demonstrates differential effects of corticosteroids on antibody-dependent cell-mediated cytotoxicity, reflecting changes in proportion of circulating effector cell populations. These changes depend on (a) the target cell employed, (b) the effector cell mediating cytotoxicity and (c) the duration of time since the last dose of corticosteroid.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGGS D. R., ATHENS J. W., CARTWRIGHT G. E., WINTROBE M. M. THE EFFECT OF ADRENAL GLUCOCORTICOSTEROIDS UPON THE CELLULAR COMPOSITION OF INFLAMMATORY EXUDATES. Am J Pathol. 1964 May;44:763–773. [PMC free article] [PubMed] [Google Scholar]
- Balow J. E., Hunninghake G. W., Fauci A. S. Corticosteroids in human lymphocyte-mediated cytotoxic reactions: effects on the kinetics of sensitization and on the cytolytic capacity of effector lymphocytes in vitro. Transplantation. 1977 Apr;23(4):322–328. [PubMed] [Google Scholar]
- Brier A. M., Chess L., Schlossman S. F. Human antibody-dependent cellular cytotoxicity. Isolation and identification of a subpopulation of peripheral blood lymphocytes which kill antibody-coated autologous target cells. J Clin Invest. 1975 Dec;56(6):1580–1586. doi: 10.1172/JCI108240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Chedid L., Juy D., Bona C. Influence of hydrocortisone on antibody-dependent lymphoid cell mediated cytotoxicity. Immunol Commun. 1974;3(5):477–487. doi: 10.3109/08820137409061127. [DOI] [PubMed] [Google Scholar]
- Chiao J. W., Good R. A. Studies of the presence of membrane receptors for complement, IgG and the sheep erythrocyte rosetting capacity on the same human lymphocytes. Eur J Immunol. 1976 Mar;6(3):157–162. doi: 10.1002/eji.1830060304. [DOI] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Cochrane A. M., Moussouros A., Thomsom A. D., Eddleston A. L., Wiiliams R. Antibody-dependent cell-mediated (K cell) cytotoxicity against isolated hepatocytes in chronic active hepatitis. Lancet. 1976 Feb 28;1(7957):441–444. doi: 10.1016/s0140-6736(76)91472-0. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Stavy L., Feldman M. Glucocorticoids and cellular immunity in vitro. Facilitation of the sensitization phase and inhibition of the effector phase of a lymphocyte anti-fibroblast reaction. J Exp Med. 1970 Dec 1;132(6):1055–1070. doi: 10.1084/jem.132.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D. C., Fauci A. S., Wolff S. M. Alternate-day prednisone. Leukocyte kinetics and susceptibility to infections. N Engl J Med. 1974 Nov 28;291(22):1154–1158. doi: 10.1056/NEJM197411282912203. [DOI] [PubMed] [Google Scholar]
- Dawkins R. L., Mastaglia F. L. Cell-mediated cytotoxicity to muscle in polymyositis. Effect of immunosuppression. N Engl J Med. 1973 Mar 1;288(9):434–438. doi: 10.1056/NEJM197303012880903. [DOI] [PubMed] [Google Scholar]
- Dimitriu A. Suppression of macrophage arming by corticosteroids. Cell Immunol. 1976 Jan;21(1):79–87. doi: 10.1016/0008-8749(76)90329-4. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., McWilliams M., Phillips-Quagliata J. M., Lamm M. E., Nussenzweig V. Immunoglobulin-bearing and complement-receptor lymphocytes constitute the same population in human peripheral blood. J Clin Invest. 1976 Jan;57(1):53–56. doi: 10.1172/JCI108268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Balow J. E., Pratt K. R. Human bone marrow lymphocytes. Cytotoxic effector cells in the bone marrow of normal individuals. J Clin Invest. 1976 Apr;57(4):826–835. doi: 10.1172/JCI108358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Human bone marrow lymphocytes. I. Distribution of lymphocyte subpopulations in the bone marrow of normal individuals. J Clin Invest. 1975 Jul;56(1):98–110. doi: 10.1172/JCI108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. II. Differential effects of in vivo hydrocortisone, prednisone and dexamethasone on in vitro expression of lymphocyte function. Clin Exp Immunol. 1976 Apr;24(1):54–62. [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Depression of cytotoxic T cell subpopulation in mice by hydrocortisone treatment. Clin Immunol Immunopathol. 1975 Jul;4(2):304–313. doi: 10.1016/0090-1229(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Modulation of polymorphonuclear leukocyte-mediated antibody-dependent cellular cytotoxicity. J Immunol. 1974 Dec;113(6):1793–1800. [PubMed] [Google Scholar]
- Garovoy M. R., Franco V., Zschaeck D., Carpenter C. B., Strom T. B., Merrill J. P. Derect lymphocyte-mediated cytotoxicity as an assay of presensitisation. Lancet. 1973 Mar 17;1(7803):573–576. doi: 10.1016/s0140-6736(73)90717-4. [DOI] [PubMed] [Google Scholar]
- Hakala T. R., Lange P. H., Castro A. E., Elliott A. Y., Fraley E. E. Antibody induction of lymphocyte-mediated cytotoxicity against human transitional-cell carcinomas of the urinary tract. N Engl J Med. 1974 Sep 26;291(13):637–641. doi: 10.1056/NEJM197409262911301. [DOI] [PubMed] [Google Scholar]
- Holm G., Hammarström S. Haemolytic activity of human blood monocytes. Lysis of human erythrocytes treated with anti-A serum. Clin Exp Immunol. 1973 Jan;13(1):29–43. [PMC free article] [PubMed] [Google Scholar]
- Holm G. Lysis of antibody-treated human erythrocytes by human leukocytes and macrophages in tissue culture. Int Arch Allergy Appl Immunol. 1972;43(5):671–682. doi: 10.1159/000230883. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren G. In vitro cytotoxicity by human lymphocytes from individuals immunized against histocompatibility antigens. I. Kinetics and specificity of the reaction. Influence of metabolic inhibitors and anti-lymphocyte serum. Clin Exp Immunol. 1970 May;6(5):661–670. [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Bonnard G. D., Sordat B., Zawodnik S. A. Antibody-dependent cell-mediated cytotoxicity: heterogeneity of effector cells in human peripheral blood. Scand J Immunol. 1975 Sep;4(5-6):487–497. doi: 10.1111/j.1365-3083.1975.tb02654.x. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- O'Toole C., Perlmann P., Wigzell H., Unsgaard B., Zetterlund C. G. Lymphocyte cytotoxicity in bladder cancer. No requirement for thymus-derived effector cells? Lancet. 1973 May 19;1(7812):1085–1088. doi: 10.1016/s0140-6736(73)90396-6. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Larsson A., Wåhlin B. Antibody dependent cytolytic effector lymphocytes (K cells) in human blood. J Reticuloendothel Soc. 1975 Apr;17(4):241–250. [PubMed] [Google Scholar]
- ROSENAU W., MOON H. D. The inhibitory effect of hydrocortisone on lysis of homologous cells by lymphocytes in vitro. J Immunol. 1962 Sep;89:422–426. [PubMed] [Google Scholar]
- Rinehart J. J., Sagone A. L., Balcerzak S. P., Ackerman G. A., LoBuglio A. F. Effects of corticosteroid therapy on human monocyte function. N Engl J Med. 1975 Jan 30;292(5):236–241. doi: 10.1056/NEJM197501302920504. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes. I. Detection of a subpopulation of murine T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Sep 1;142(3):611–621. doi: 10.1084/jem.142.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Tilney N. L., Carpenter C. B., Busch G. J. Identity and cytotoxic capacity of cells infiltrating renal allografts. N Engl J Med. 1975 Jun 12;292(24):1257–1263. doi: 10.1056/NEJM197506122922402. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Hensen S. A., Vincent M. M., Rola-Pleszcynski M., Walser J. B., Bellanti J. A. Effect of hydrocortisone on in vitro cellular immunity to viruses in man. Clin Immunol Immunopathol. 1975 Jan;3(3):363–368. doi: 10.1016/0090-1229(75)90023-9. [DOI] [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel M. L., Ritts R. E., Jr, Taswell H. F., Danadio J. V., Jr, Woods J. E. Cellular immunity after intravenous administration of methylprednisolone. J Lab Clin Med. 1974 Mar;83(3):383–392. [PubMed] [Google Scholar]
- Williams T. W., Granger G. A. Lymphocyte in vitro cytotoxicity: correlation of derepression with release of lymphotoxin from human lymphocytes. J Immunol. 1969 Aug;103(2):170–178. [PubMed] [Google Scholar]
- Yu D. T., Clements P. J., Paulus H. E., Peter J. B., Levy J., Barnett E. V. Human lymphocyte subpopulations. Effect of corticosteroids. J Clin Invest. 1974 Feb;53(2):565–571. doi: 10.1172/JCI107591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeijlemaker W. P., Roos M. T., Schellekens P. T., Eijsvoogel V. P. Antibody-dependent human lymphocytotoxicity: a micro assay system. Eur J Immunol. 1975 Aug;5(8):579–584. doi: 10.1002/eji.1830050815. [DOI] [PubMed] [Google Scholar]


