Abstract
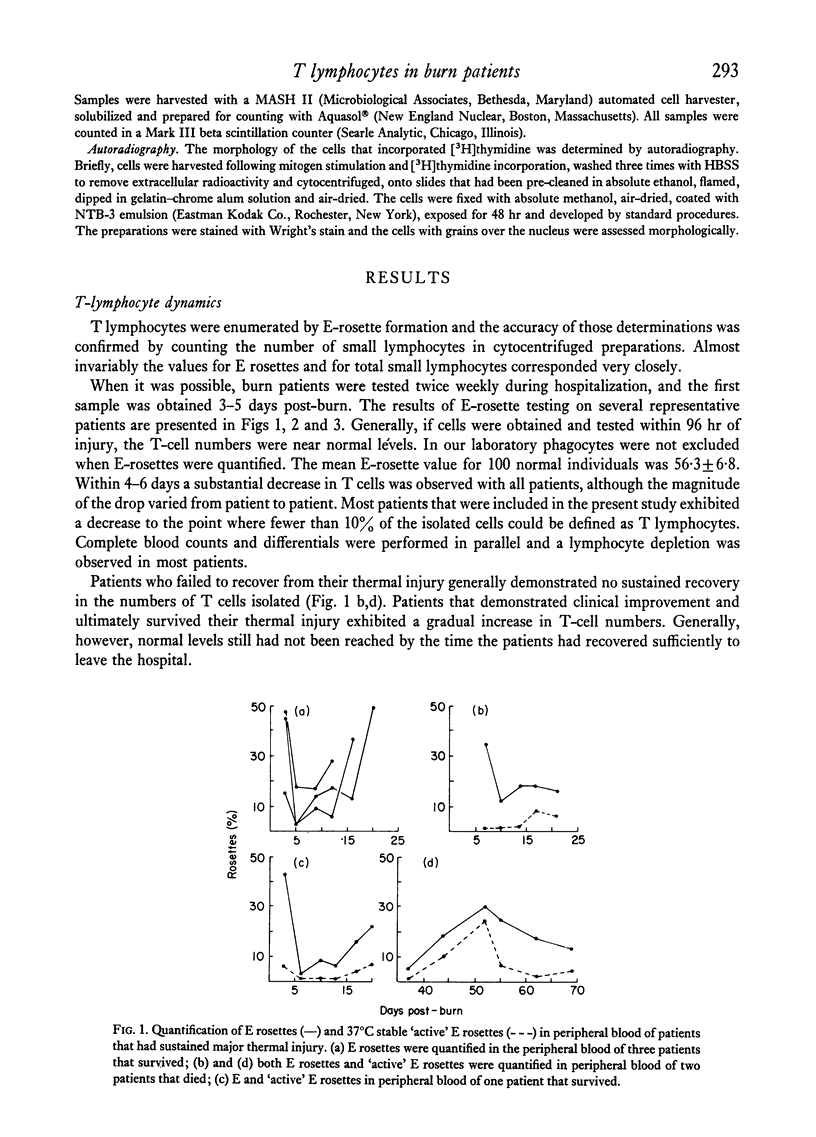
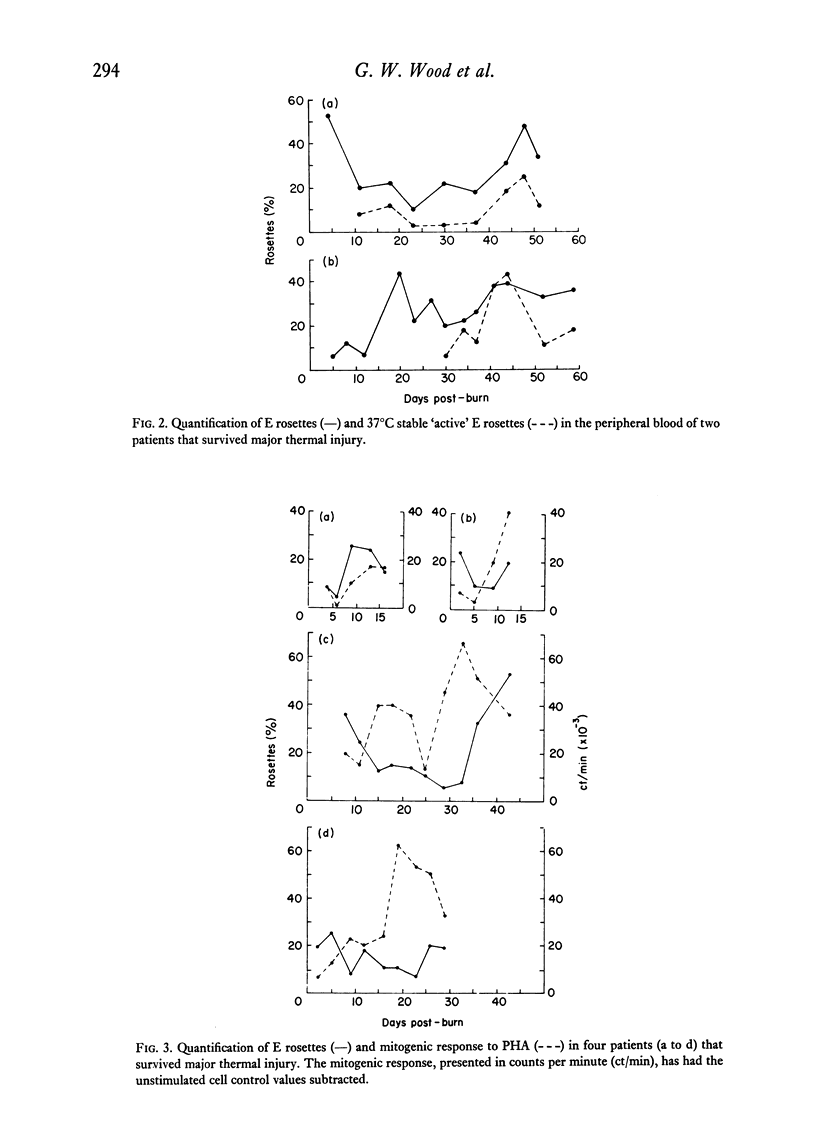
The study was designed to evaluate T-lymphocyte function in relation to the numbers of T cells present in patients that had sustained major thermal injury. Also, it was designed to determine the dynamics of total T cells and the T-cell subpopulation that formed `active' E rosettes in separated cell populations. The primary observations were: (a) within 10 days of injury a significant depression of T-cell numbers in separated cell populations occurred, which was paralleled by a decrease in T-cell function. Thus the immunosuppression that has been observed in burn patients appears to have been related to decreases in the numbers of T cells, rather than to any dysfunction at the level of the individual T cell; (b) although T-cell numbers remained depressed, in some patients mitogen responses returned to very high levels, suggesting the possibility that either a highly responsive T-cell subpopulation was selected or that there was a depletion of suppressor cells; (c) in patients that survived there was a gradual return of T lymphocyte numbers to normal levels; and (d) the decreases in the total T-cell population were accompanied by a depletion of the T cells that formed `active' (37°C stable) rosettes, and the recovery of normal T-cell numbers was accompanied by a disproportionate increase in `active' rosette-forming cells. The high numbers of `active' rosettes during the recruitment of new T cells suggested that this T-cell subpopulation may represent cells recently arrived in the peripheral blood from the precursor pools.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borella L., Sen L. E receptors on blasts from untreated acute lymphocytic leukemia (ALL): comparison of temperature dependence of E rosettes formed by normal and leukemic lymphoid cells. J Immunol. 1975 Jan;114(1 Pt 1):187–190. [PubMed] [Google Scholar]
- Casson P., Solowey A. C., Converse J. M., Rapaport F. T. Delayed hypersensitivity status of burned patients. Surg Forum. 1966;17:268–270. [PubMed] [Google Scholar]
- Daniels J. C., Cobb E. K., Lynch J. B., Lewis S. R., Larson D. L., Ritzmann S. E. Altered nucleic acid synthesis in lymphocytes from patients with thermal burns. Surg Gynecol Obstet. 1970 May;130(5):783–788. [PubMed] [Google Scholar]
- Daniels J. C., Sakai H., Cobb E. K., Lewis S. R., Larson D. L., Ritzmann S. E. Evaluation of lymphocyte reactivity studies in patients with thermal burns. J Trauma. 1971 Jul;11(7):595–601. doi: 10.1097/00005373-197107000-00011. [DOI] [PubMed] [Google Scholar]
- Fudenberg H. H. Genetically determined immune deficiency as the predisposing cause of "autoimmunity" and lymphoid neoplasia. Am J Med. 1971 Sep;51(3):295–298. doi: 10.1016/0002-9343(71)90263-4. [DOI] [PubMed] [Google Scholar]
- Garrioch D. B., Good R. A., Gatti R. A. Lymphocyte response to P.H.A. in patients with non-lymphoid tumours. Lancet. 1970 Mar 21;1(7647):618–618. doi: 10.1016/s0140-6736(70)91658-2. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. E., Sheil A. G. E-rosette forming cell numbers in the blood of human renal allograft recipients. Clin Exp Immunol. 1977 Mar;27(3):454–463. [PMC free article] [PubMed] [Google Scholar]
- Leguit P., Jr, Meinesz A., Zeijlemaker W. P., Schellekens P. T., Eijsvoogel V. P. Immunological studies in burn patients. I. Lymphocyte transformation in vitro. Int Arch Allergy Appl Immunol. 1973;44(1):101–121. doi: 10.1159/000230921. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Epstein W., Fudenberg H. H. Immunopathologic changes in patients with cutaneous malignant melanoma following intratumoral inoculation BCG: correlation with cell-mediated immunity. Int J Cancer. 1974 Sep 15;14(3):401–416. doi: 10.1002/ijc.2910140315. [DOI] [PubMed] [Google Scholar]
- Mahler D., Batchelor J. R. Phytohaemagglutinin transformation of lymphocytes in burned patients. Transplantation. 1971 Nov;12(5):409–411. doi: 10.1097/00007890-197111000-00015. [DOI] [PubMed] [Google Scholar]
- Rapaport F. T., Milgrom F., Kano K., Gesner B., Solowey A. C., Casson P., Silverman H. I., Converse J. M. Immunologic sequelae of thermal injury. Ann N Y Acad Sci. 1968 Aug 14;150(3):1004–1008. doi: 10.1111/j.1749-6632.1968.tb14753.x. [DOI] [PubMed] [Google Scholar]
- Sakai H., Daniels J. C., Lewis S. R., Lynch J. B., Larson D. L., Ritzmann S. E. Reversible alterations of nucleic acid synthesis in lymphocytes after thermal burns. J Reticuloendothel Soc. 1972 Jan;11(1):19–28. [PubMed] [Google Scholar]
- Wybran J., Belohradsky B. H., Fudenberg H. H. Unmasking by antimetabolites of receptors for sheep red blood cells on human lymphocytes. Cell Immunol. 1974 Dec;14(3):359–365. doi: 10.1016/0008-8749(74)90185-3. [DOI] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells. N Engl J Med. 1973 May 17;288(20):1072–1073. doi: 10.1056/NEJM197305172882011. [DOI] [PubMed] [Google Scholar]


