Abstract
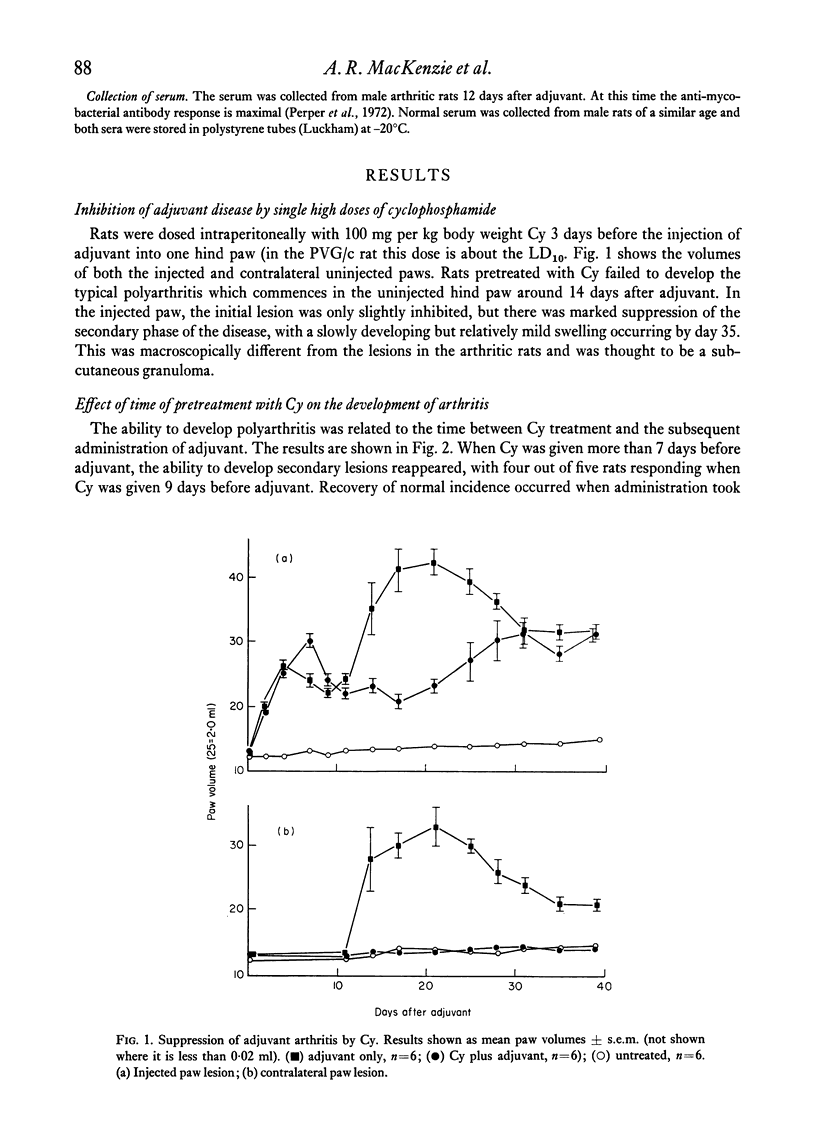
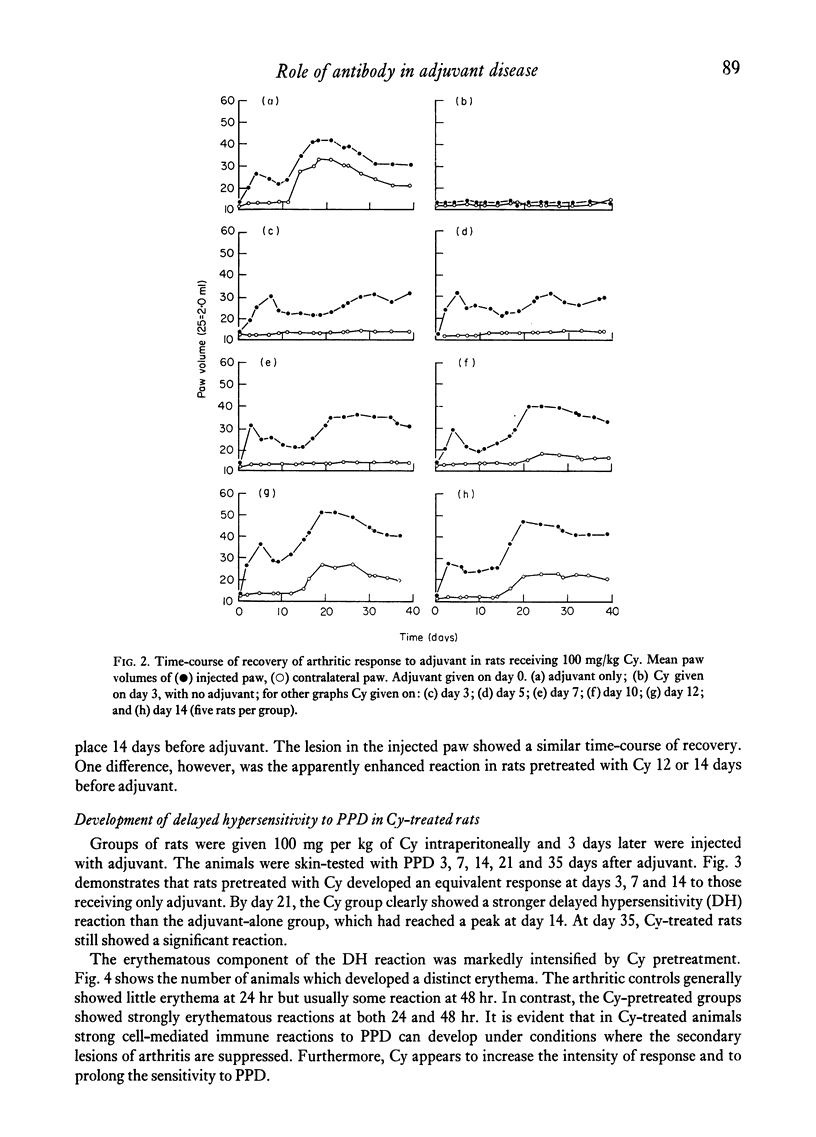
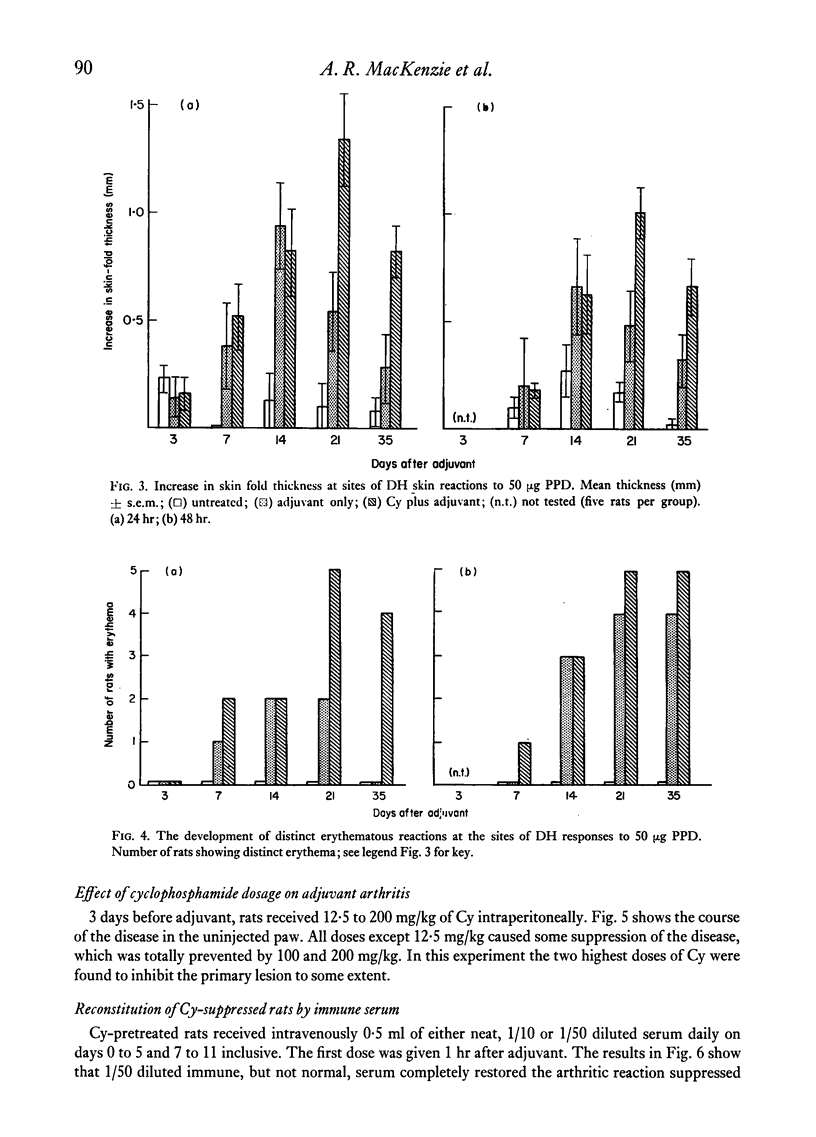
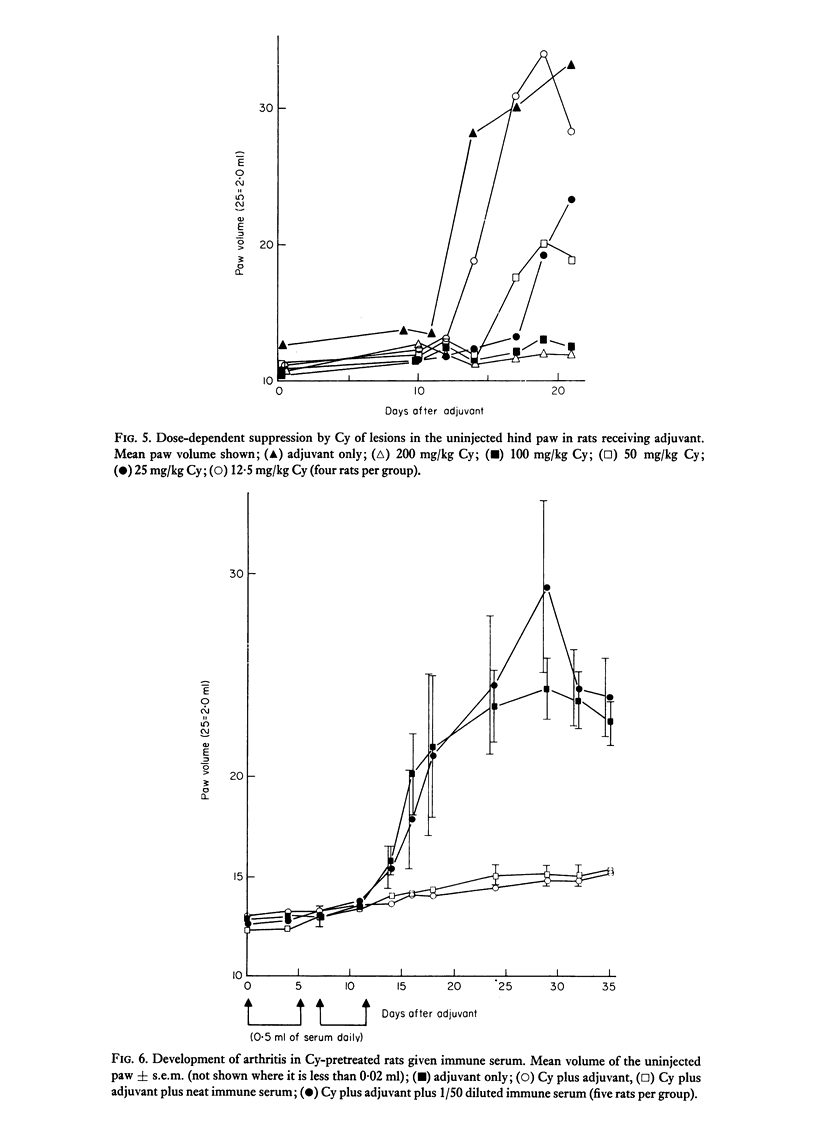
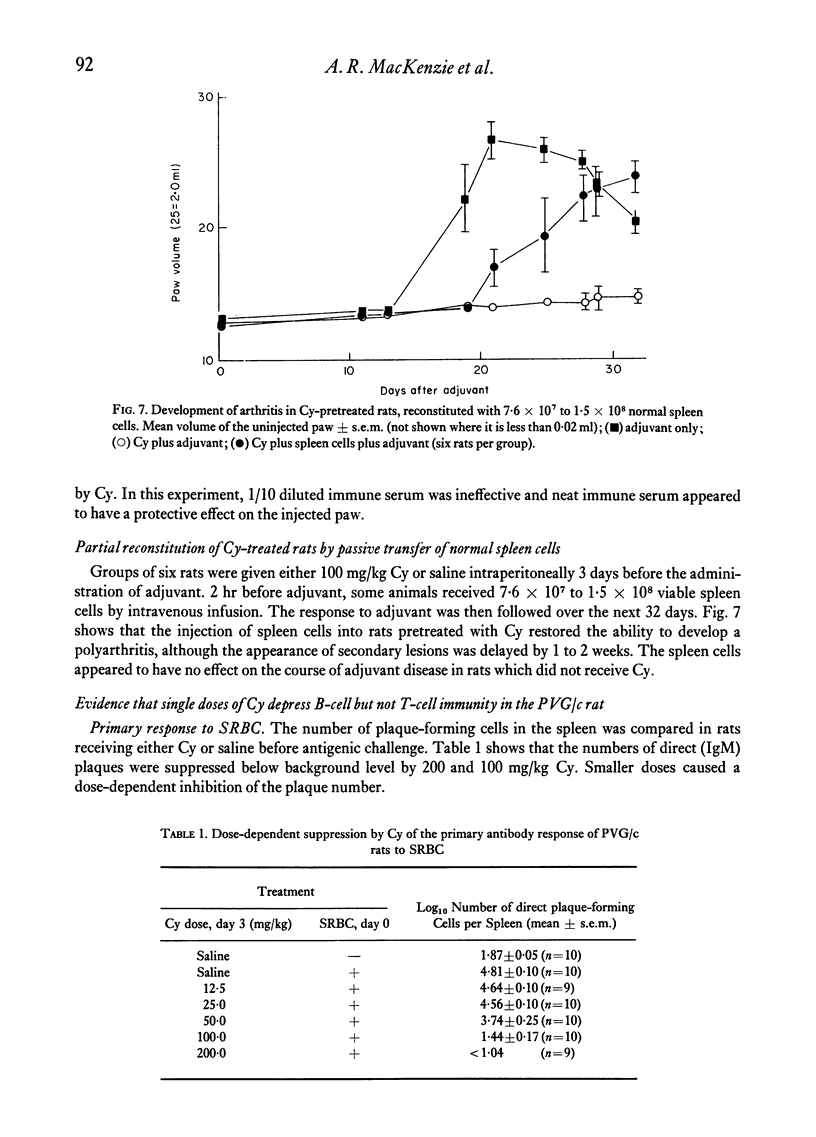
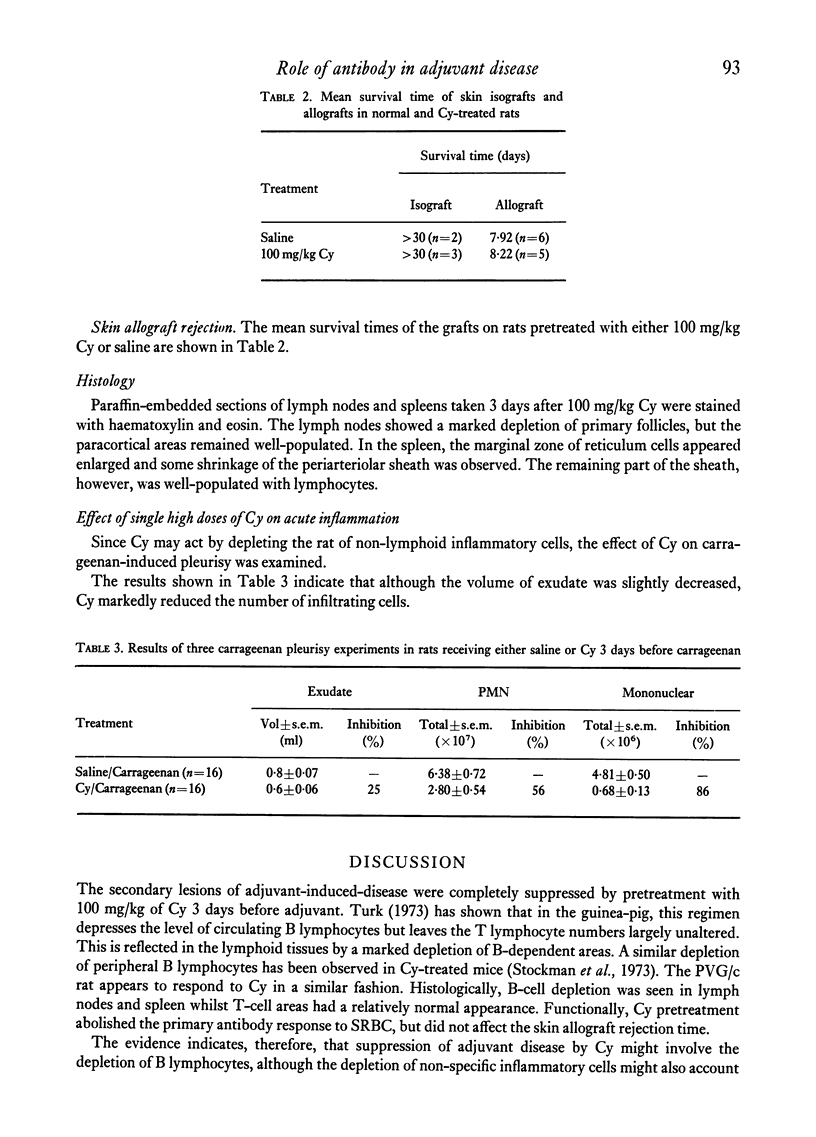
Cyclophosphamide (Cy), given intraperitoneally at a dose of 100 mg per kg body weight 3 days before adjuvant, was found to abolish the development of adjuvant disease in the PVG/c rat. This treatment, however, enhanced the delayed hypersensitivity responses to purified protein derivative of tuberculin (PPD) developed by these animals. Lower doses of Cy caused a partial inhibition of arthritis which was dose-related. When the time between giving Cy and the injection of adjuvant was increased, a gradual time-dependent recovery of the response was observed. The arthritic response was restored by the passive transfer of 7.6 x 10(7) to 1.5 x 10(8) normal syngeneic spleen cells, although the development of secondary lesions was delayed by 7-14 days. The response could also be restored by the transfer of small amounts of serum from arthritic, but not normal, rats. Large amounts of serum failed to restore the response. Additional evidence that pretreatment with Cy preferentially depleted the B lymphocytes was obtained by the histological examination of the lymphoid tissue. It was also shown that the primary antibody response to sheep erythrocytes was abolished by Cy, but that skin allograft rejection was unaffected. A partial inhibition of the acute inflammatory reaction to carrageenan was observed 3 days after giving Cy. It is suggested that the pathogenesis of adjuvant arthritis involves an immune complex-mediated phase, whihc initiates the joint lesions. Once these lesions have formed, cell-mediated immune mechanisms predominate in the development of the disease. It is not known whether the persistence of immune complexes is necessary to maintain the lesions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTEIN N. A., WAKSMAN B. H. THE PATHOGENESIS OF ADJUVANT DISEASE IN THE RAT. I. A HISTOLOGIC STUDY OF EARLY LESIONS IN THE JOINTS AND SKIN. Yale J Biol Med. 1964 Dec;37:177–194. [PMC free article] [PubMed] [Google Scholar]
- Berry H., Willoughby D. A., Giroud J. P. Evidence for an endogenous antigen in the adjuvant arthritic rat. J Pathol. 1973 Dec;111(4):229–238. doi: 10.1002/path.1711110403. [DOI] [PubMed] [Google Scholar]
- Billingham M. E., Gordon A. H. The role of the acute phase reaction in inflammation. Agents Actions. 1976 Feb;6(1-3):195–200. doi: 10.1007/BF01972208. [DOI] [PubMed] [Google Scholar]
- Cot deaths. Lancet. 1965 Oct 30;2(7418):889–889. [PubMed] [Google Scholar]
- Currey H. L. A comparison of immunosuppressive and anti-inflammatory agents in the rat. Clin Exp Immunol. 1971 Dec;9(6):879–887. [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M., Giroud J. P., Willoughby D. A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971 May;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- Glenn E. M., Gray J. Adjuvant-induced polyarthritis in rats: biologic and histologic background. Am J Vet Res. 1965 Sep;26(114):1180–1194. [PubMed] [Google Scholar]
- Hollingsworth J. W., Greenberg D. S., Dawson M. Adjuvant arthritis in T lymphocyte depleted rats. Proc Soc Exp Biol Med. 1976 Jun;152(2):183–185. doi: 10.3181/00379727-152-39356. [DOI] [PubMed] [Google Scholar]
- ISAKOVIC K., WAKSMAN B. H. EFFECT OF SENSITIZATION TO BSA ON ADJUVANT DISEASE IN NORMAL AND NEONATALLY THYMECTOMIZED RATS. Proc Soc Exp Biol Med. 1965 Jul;119:676–678. doi: 10.3181/00379727-119-30269. [DOI] [PubMed] [Google Scholar]
- JONES R. S., WARD J. R. Studies on adjuvant-induced polyarthritis in rats. II. Histogenesis of joint and visceral lesions. Arthritis Rheum. 1963 Feb;6:23–35. doi: 10.1002/art.1780060104. [DOI] [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. I. Evidence for the regulatory function of thymus-derived cells in the induction of the disease. J Immunol. 1976 Nov;117(5 PT2):1878–1882. [PubMed] [Google Scholar]
- Koga T., Pearson C. M. Immunogenicity and arthritogenicity in the rat of an antigen from Mycobacterium tuberculosis wax D. J Immunol. 1973 Aug;111(2):599–608. [PubMed] [Google Scholar]
- Kourounakis L., Nelson R. A., Jr, Kupusta M. A. The effect of a cobra venom factor on complement and adjuvant-induced disease in rats. Arthritis Rheum. 1973 Jan-Feb;16(1):71–76. doi: 10.1002/art.1780160111. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Byrd W. J. Letter: Experimental arthritis in thymectomized rats with an impaired humoral immune response. Nature. 1973 Jul 6;244(5410):38–40. doi: 10.1038/244038a0. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Oronsky A. L., Blancuzzi V. Antibody to mycobacterial antigens in adjuvant arthritis (AA). I. Relationship to disease and delayed hypersensitivity. Arthritis Rheum. 1972 May-Jun;15(3):283–292. doi: 10.1002/art.1780150310. [DOI] [PubMed] [Google Scholar]
- Quagliata F., Phillips-Quagliata J. M. Competence of thoracic duct cells in the transfer of adjuvant disease and delayed hypersensitivity. Evidence that mycobacterial components are required for the successful transfer of the disease. Cell Immunol. 1972 Jan;3(1):78–87. doi: 10.1016/0008-8749(72)90228-6. [DOI] [PubMed] [Google Scholar]
- Turk J. L. Evidence for a preferential effect of cyclophosphamide on B-cells. Proc R Soc Med. 1973 Aug;66(8):805–808. [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., PEARSON C. M., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J Immunol. 1960 Oct;85:403–417. [PubMed] [Google Scholar]
- WAKSMAN B. H., WENNERSTEN C. PASSIVE TRANSFER OF ADJUVANT ARTHRITIS IN RATS WITH LIVING LYMPHOID CELLS OF SENSITIZED DONORS. Int Arch Allergy Appl Immunol. 1963;23:129–139. doi: 10.1159/000229412. [DOI] [PubMed] [Google Scholar]
- WARD J. R., CLOUD R. S., KRAWITT E. L., JONES R. S. STUDIES ON ADJUVANT-INDUCED POLYARTHRITIS IN RATS. 3. THE EFFECT OF "IMMUNOSUPPRESSIVE AGENTS" ON ARTHRITIS AND TUBERCULIN HYPERSENSITIVITY. Arthritis Rheum. 1964 Dec;7:654–661. doi: 10.1002/art.1780070605. [DOI] [PubMed] [Google Scholar]
- Whitehouse D. J., Whitehouse M. W., Pearson C. M. Passive transfer of adjuvant-induced arthritis and allergic encephalomyelitis in rats using thoracic duct lymphocytes. Nature. 1969 Dec 27;224(5226):1322–1322. doi: 10.1038/2241322a0. [DOI] [PubMed] [Google Scholar]


