Abstract
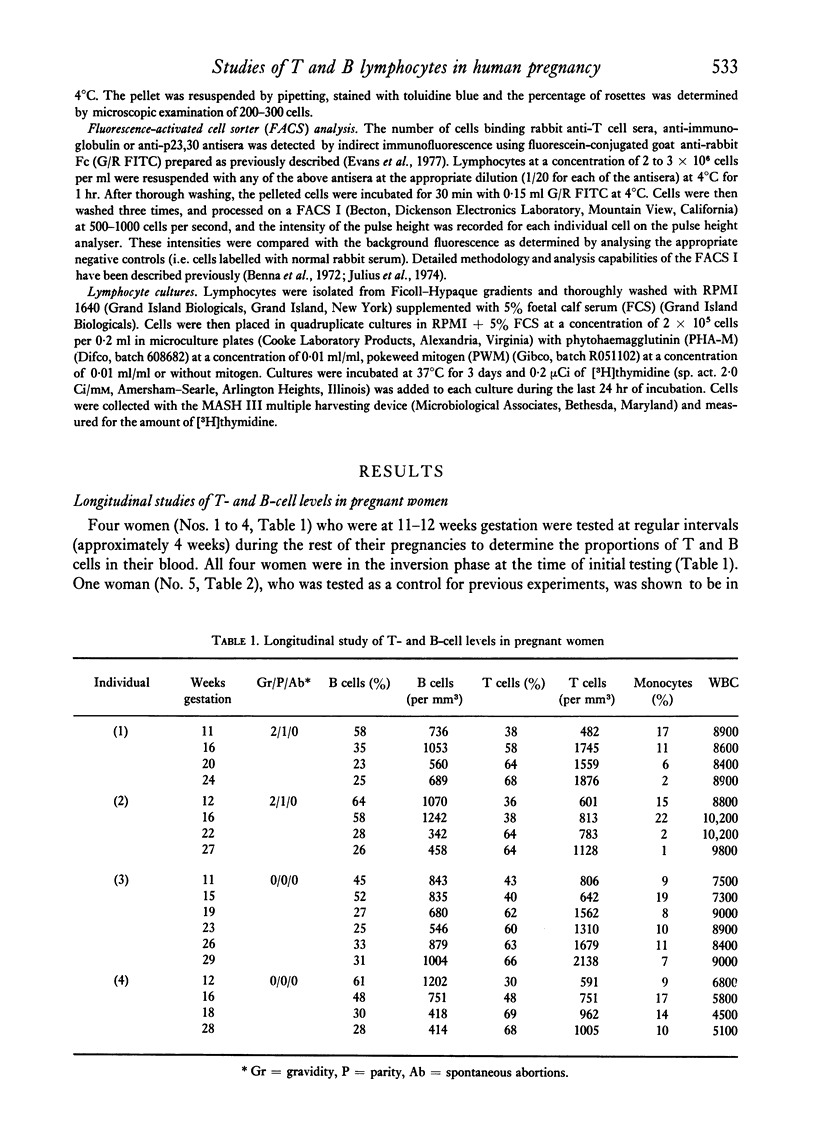
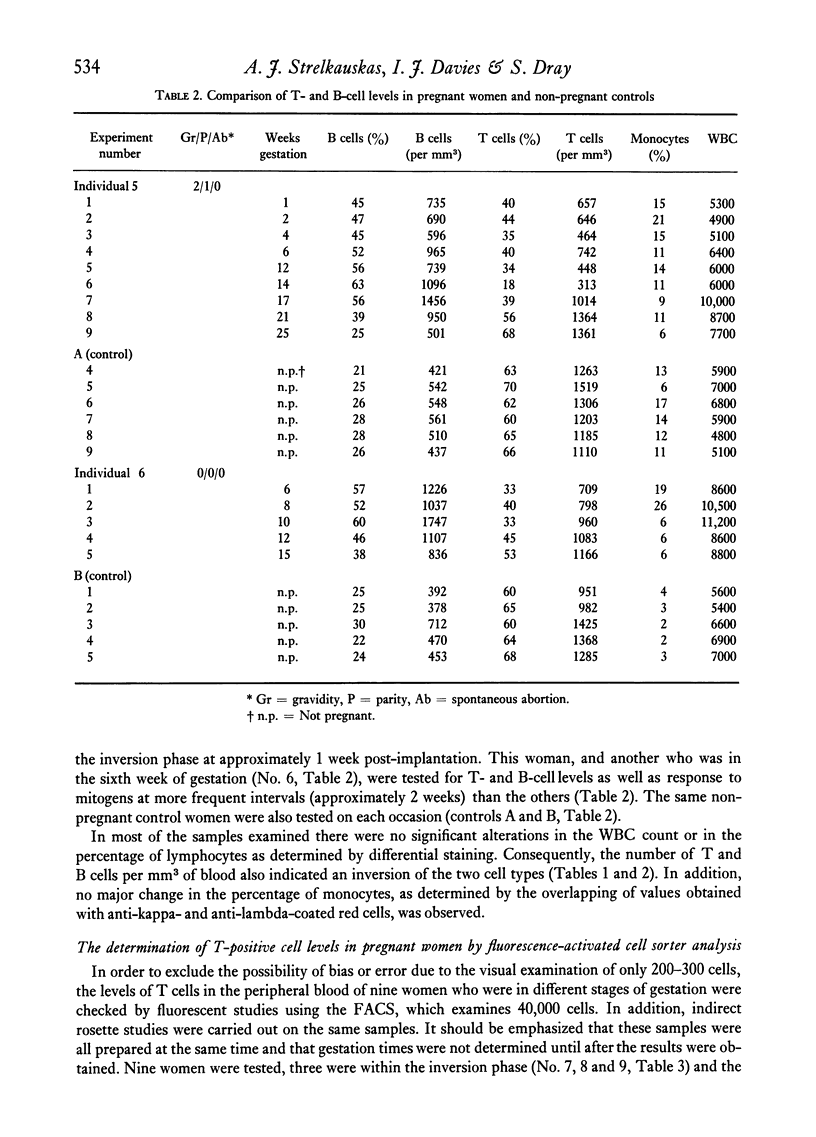
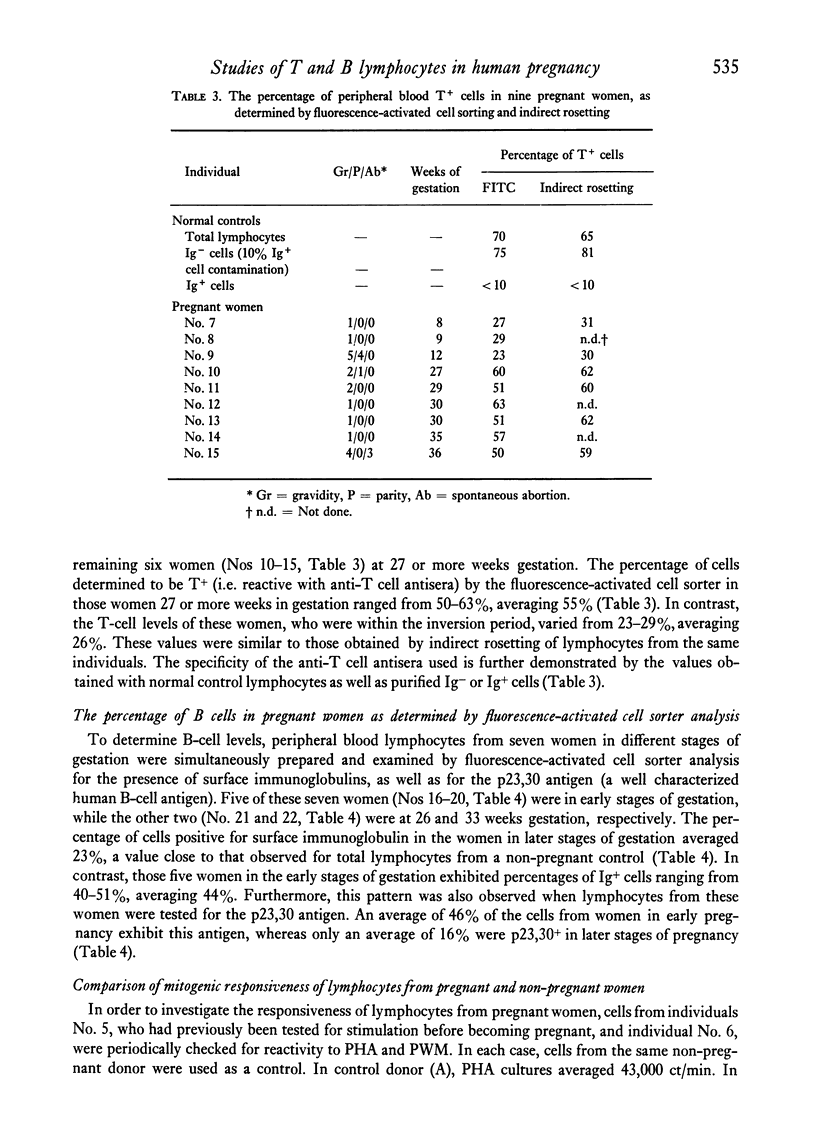
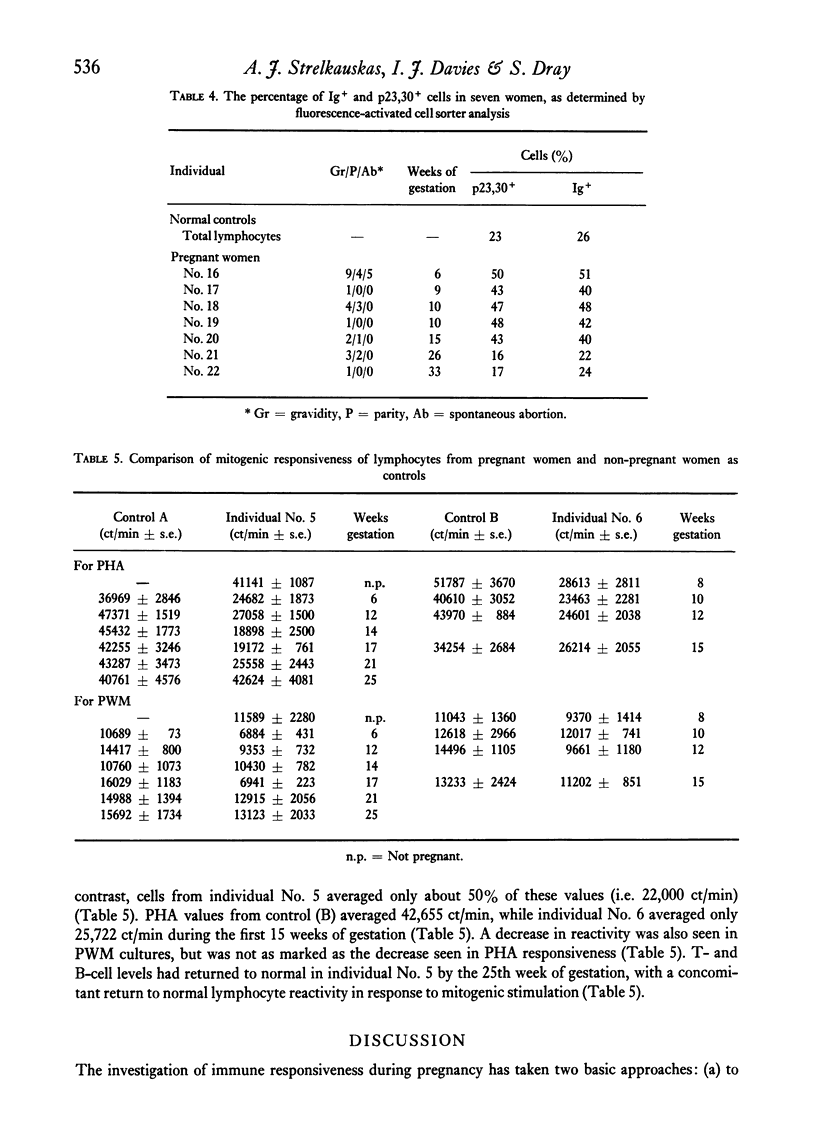
Altered blood levels of T and B lymphocytes were found in the first half of human pregnancy. A total of twenty-two women were tested, using direct or indirect rosetting assays or the fluorescence-activated cell sorter, to determine the levels of peripheral blood T and B cells. In all cases, an inversion of T- and B-cell levels was observed, i.e. T-cell levels were decreased and B-cell levels (as measured by the presence of surface immunoglobulin or the presence of B-cell surface antigens) were increased. This inversion was exhibited as early as 1 week post-implantation. Lymphocytes from two fo the women were also examined for stimulation with phytohaemagglutinin (PHA) and pokeweed mitogen (PWM) at intervals during gestation, and the amount of [3H]thymidine uptake was compared to that of two non-pregnant women tested at each interval. The values obtained for the pregnant women with PHA were markedly lower, and with pokeweed mitogen slightly lower, than those of non-pregnant controls. However, the PHA and PWM values in the pregnant women returned to levels similar to those of the nonpregnant women shortly after the T- and B-cell levels returned to normal. Thus the decrease in the response of the lymphocytes to mitogens during early pregnancy appears to parallel the numerical deficiency of T cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beer A. E., Scott J. R., Billingham R. E. Histoincompatibility and maternal immunological status as determinants of fetoplacental weight and litter size in rodents. J Exp Med. 1975 Jul 1;142(1):180–196. doi: 10.1084/jem.142.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham R. E. The transplantation biology of mammalian gestation. Am J Obstet Gynecol. 1971 Oct 15;111(4):469–483. doi: 10.1016/0002-9378(71)90463-7. [DOI] [PubMed] [Google Scholar]
- Bonneau M., Latour M., Revillard J. P., Robert M., Traeger J. Blocking antibodies eluted from human placenta. Transplant Proc. 1973 Mar;5(1):589–592. [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Brent R. L. Production of congenital malformations using tissue antisera. 3. Placental antiserum. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1024–1029. doi: 10.3181/00379727-125-32267. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Schiff R. I., Amos D. B. Blocking of autologous and homologous leukocyte responses by human alloimmune plasmas: a possible in vitro correlate of enhancement. J Immunol. 1972 Jan;108(1):34–44. [PubMed] [Google Scholar]
- Bulmer R., Hancock K. W. Depletion of circulating T lymphocytes in pregnancy. Clin Exp Immunol. 1977 May;28(2):302–305. [PMC free article] [PubMed] [Google Scholar]
- Carr M. C., Stites D. P., Fudenberg H. H. Cellular immune aspects of the human fetal maternal relationship. II. In vitro response of gravida lymphocytes to phytohemagglutinin. Cell Immunol. 1973 Sep;8(3):448–454. doi: 10.1016/0008-8749(73)90136-6. [DOI] [PubMed] [Google Scholar]
- Comings D. E. Lymphocyte transformation in response to phytohemagglutinin during and following a pregnancy. Am J Obstet Gynecol. 1967 Jan 15;97(2):213–217. doi: 10.1016/0002-9378(67)90543-1. [DOI] [PubMed] [Google Scholar]
- Dodson M. G., Kerman R. H., Lange C. F., Stefani S. S., O'Leary J. A. T and B cells in pregnancy. Obstet Gynecol. 1977 Mar;49(3):299–302. [PubMed] [Google Scholar]
- Evans R. L., Breard J. M., Lazarus H., Schlossman S. F., Chess L. Detection, isolation, and functional characterization of two human T-cell subclasses bearing unique differentiation antigens. J Exp Med. 1977 Jan 1;145(1):221–233. doi: 10.1084/jem.145.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R., St Hill C. A., Govan A. J., Ralfs I. G., Gurney F. J., Denye V. Immunological responses in pregnancy and survival of fetal homograft. Br Med J. 1972 Jul 15;3(5819):150–152. doi: 10.1136/bmj.3.5819.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti R. A., Yunis E. J., Good R. A. Characterization of a serum inhibitor of MLC reactions. Clin Exp Immunol. 1973 Mar;13(3):427–437. [PMC free article] [PubMed] [Google Scholar]
- HULKA J. F., BRINTON V., SCHAAF J., BANEY C. Appearance of antibodies to trophoblast during the postpartum period in normal human pregnancies. Nature. 1963 May 4;198:501–502. doi: 10.1038/198501a0. [DOI] [PubMed] [Google Scholar]
- HULKA J. F., HSU K. C., BEISER S. M. Antibodies to trophoblasts during the post-partum period. Nature. 1961 Jul 29;191:510–511. doi: 10.1038/191510a0. [DOI] [PubMed] [Google Scholar]
- Knobloch V., Jouja V., Svobodová M. Morphological changes in maternal lymphocytes in pregnancy. Br J Obstet Gynaecol. 1975 Feb;82(2):146–150. doi: 10.1111/j.1471-0528.1975.tb02213.x. [DOI] [PubMed] [Google Scholar]
- Maroni E. S., Parrott D. M. Progressive increase in cell-mediated immunity against paternal transplantation antigens in parous mice after multiple pregnancies. Clin Exp Immunol. 1973 Feb;13(2):253–262. [PMC free article] [PubMed] [Google Scholar]
- McCormick J. N., Faulk W. P., Fox H., Fudenberg H. H. Immunohistological and elution studies of the human placenta. J Exp Med. 1971 Jan 1;133(1):1–18. doi: 10.1084/jem.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence H., Petty W. M., Rocklin R. E. Suppression of maternal responsiveness to paternal antigen by maternal plasma. J Immunol. 1975 Jan;114(1 Pt 2):525–528. [PubMed] [Google Scholar]
- Purtilo D. T., Hallgren H. M., Yunis E. J. Depressed maternal lymphocyte response to phytohaemagglutinin in human pregnancy. Lancet. 1972 Apr 8;1(7754):769–771. doi: 10.1016/s0140-6736(72)90522-3. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Zuckerman J. E., Alpert E., David J. R. Effect of multiparity on human maternal hypersensitivity to foetal antigen. Nature. 1973 Jan 12;241(5385):130–131. doi: 10.1038/241130a0. [DOI] [PubMed] [Google Scholar]
- Sjögren H. O., Hellström I., Bansal S. C., Hellström K. E. Suggestive evidence that the "blocking antibodies" of tumor-bearing individuals may be antigen--antibody complexes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1372–1375. doi: 10.1073/pnas.68.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkauskas A. J., Wilson B. S., Dray D., Dodson M. Inversion of levels of human T and B cells in early pregnancy. Nature. 1975 Nov 27;258(5533):331–332. doi: 10.1038/258331a0. [DOI] [PubMed] [Google Scholar]
- Thiede H. A., Choate J. W., Dyre S. Pregnancy and the lymphocyte. Am J Obstet Gynecol. 1968 Nov 1;102(5):642–653. doi: 10.1016/0002-9378(68)90378-5. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Cell-mediated anti-embryo cytotoxicity in human pregnancy. Clin Exp Immunol. 1976 Mar;23(3):462–470. [PMC free article] [PubMed] [Google Scholar]
- WOODRUFF M. F. Transplantation immunity and the immunological problem of pregnancy. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):68–75. doi: 10.1098/rspb.1958.0005. [DOI] [PubMed] [Google Scholar]
- Watkins S. M. Lymphocyte response to phytohaemagglutin in pregnancy. J Obstet Gynaecol Br Commonw. 1972 Nov;79(11):990–993. doi: 10.1111/j.1471-0528.1972.tb11876.x. [DOI] [PubMed] [Google Scholar]
- Youtananukorn V., Matangkasombut P. Human maternal cell mediated immune reaction to placental antigens. Clin Exp Immunol. 1972 Aug;11(4):549–556. [PMC free article] [PubMed] [Google Scholar]


