Abstract
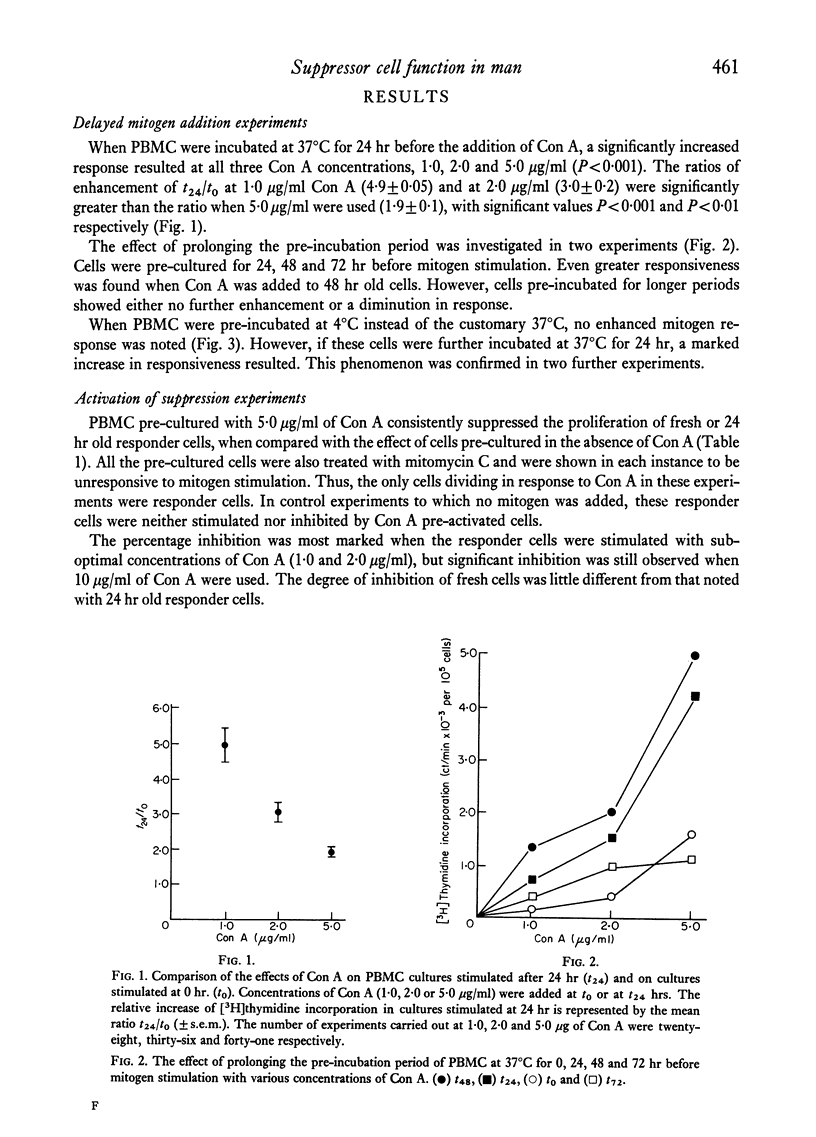
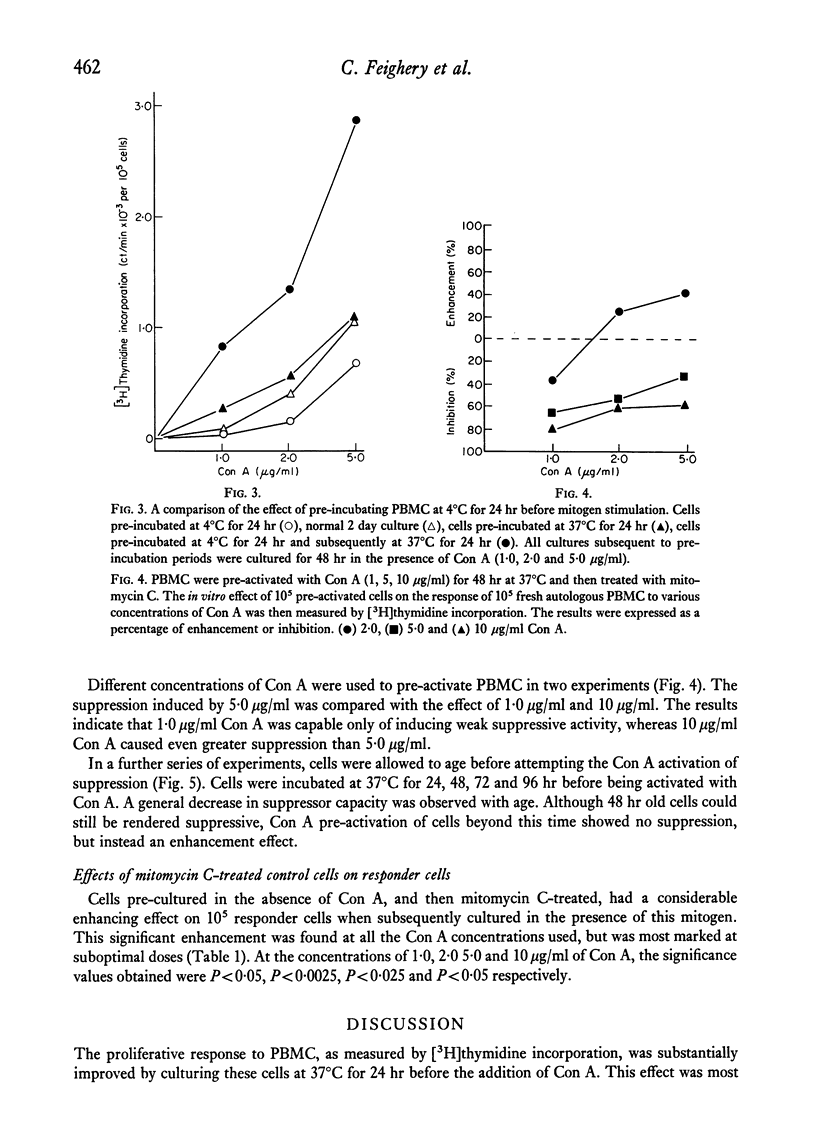
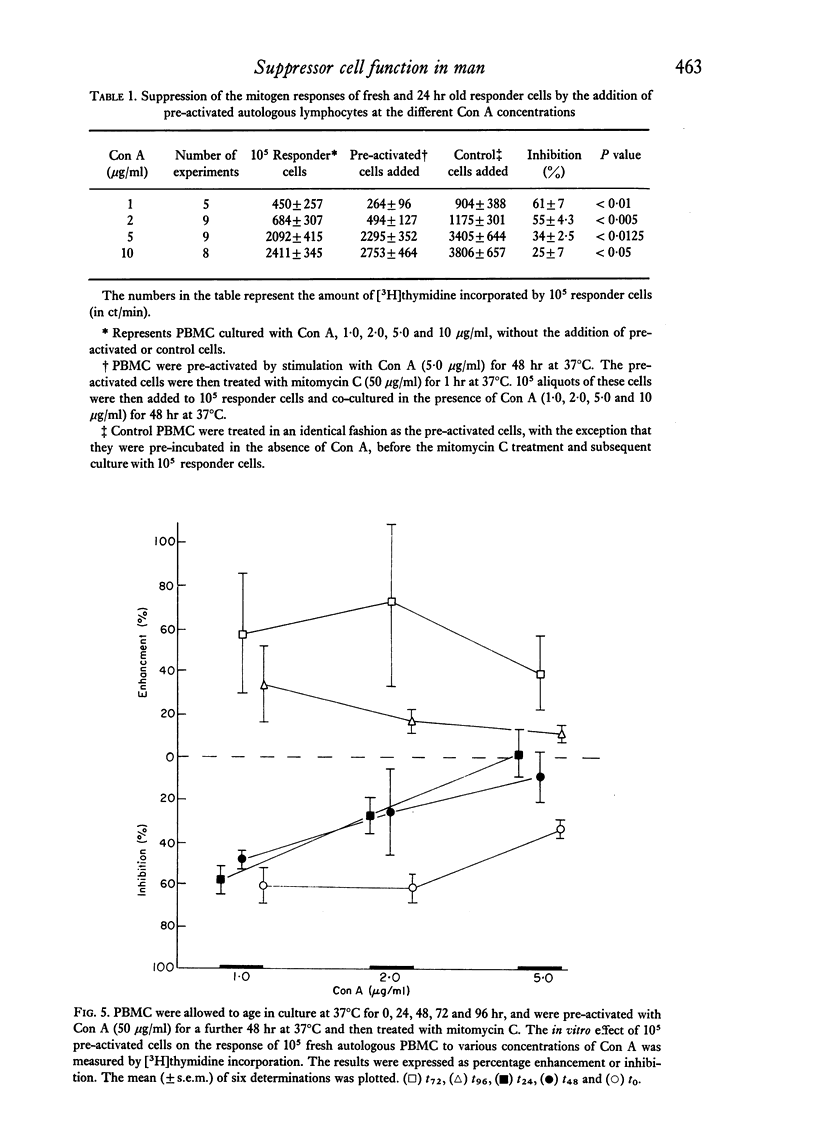
Peripheral blood mononuclear cells (PBMC) from normal donors, pre-cultured at 37 degrees C for 24 hr before the addition of mitogen, demonstrated an enhanced proliferative response. This may be due to the loss of a subpopulation of suppressor cells during the incubation period. Still further enhancement was observed when pre-culturing was prolonged for 48 hr, while cells pre-incubated at 4 degrees C showed no increased responsiveness. Concanavalin A (Con A) pre-activated PBMC supressed the mitogen response of responder cells. More marked suppression was observed when the concentration of Con A used to induce the suppressor cells was increased. It was not possible to activate suppressor function in cells which had been kept in vitro for longer than 48 hr. These findings support the concept of the existence and function of suppressor cells, and that the suppressive influence is short-lived in vitro culture.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Crumpton M. J. Phytohemagglutinin-lymphocyte interaction. Characterization of binding sites on pig lymphocytes for 125 I-labelled phytohemagglutinin. Exp Cell Res. 1973 Apr;78(2):271–278. doi: 10.1016/0014-4827(73)90069-4. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Suppressor T cells. Transplant Rev. 1975;26:39–55. doi: 10.1111/j.1600-065x.1975.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. II. Suppression of mixed lymphocyte reaction by thymus-dependent adherent cells. J Immunol. 1974 Jul;113(1):140–144. [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Rankin B., Thorsby E. Production of blastogenic factor by mitomycin-treated cells in mixed lymphocyte cultures. Transplantation. 1974 Mar;17(3):323–325. doi: 10.1097/00007890-197403000-00018. [DOI] [PubMed] [Google Scholar]
- Hubert C., Delespesse G., Govaerts A. Concanavalin A-activated suppressor cells in normal human peripheral blood lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):95–98. [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. M. Effects of concanavalin A on the in vitro responses of mouse spleen cells to T-dependent and T-independent antigens. J Immunol. 1975 Jan;114(1 Pt 2):365–370. [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]


