Abstract
Excessive uptake of atherogenic lipoproteins such as modified low-density lipoprotein complexes by vascular macrophages leads to foam cell formation, a critical step in atherogenesis. Cholesterol efflux mediated by high-density lipoproteins (HDL) constitutes a protective mechanism against macrophage lipid overloading. The molecular mechanisms underlying this reverse cholesterol transport process are currently not fully understood. To identify effector proteins that are involved in macrophage lipid uptake and release, we searched for genes that are regulated during lipid influx and efflux in human macrophages using a differential display approach. We report here that the ATP-binding cassette (ABC) transporter ABCG1 (ABC8) is induced in monocyte-derived macrophages during cholesterol influx mediated by acetylated low-density lipoprotein. Conversely, lipid efflux in cholesterol-laden macrophages, mediated by the cholesterol acceptor HDL3, suppresses the expression of ABCG1. Immunocytochemical and flow cytometric analyses revealed that ABCG1 is expressed on the cell surface and in intracellular compartments of cholesterol-laden macrophages. Inhibition of ABCG1 protein expression using an antisense strategy resulted in reduced HDL3-dependent efflux of cholesterol and choline-phospholipids. In a comprehensive analysis of the expression and regulation of all currently known human ABC transporters, we identified an additional set of ABC genes whose expression is regulated by cholesterol uptake or HDL3-mediated lipid release, suggesting a potential function for these transporters in macrophage lipid homeostasis. Our results demonstrating a regulator function for ABCG1 in cholesterol and phospholipid transport define a biologic activity for ABC transporters in macrophages.
Cholesterol homeostasis in macrophages involves a balance between lipid influx and efflux. Deposition of excessive amounts of cholesteryl ester in macrophages of the vascular wall leading to foam cell formation is a key event in atherogenesis (1). The acquisition of cholesterol by macrophages is mediated by receptor-dependent and receptor-independent processes involving both normal and modified lipoproteins. A number of macrophage cell surface receptors have been identified that can bind chemically modified low-density lipoprotein (LDL) and include the class A scavenger receptors, CLA-1, class BI scavenger receptor, FcγRII (CD32), CD36, and CD68 (for review, see ref. 2).
Efficient reverse cholesterol transport from peripheral cells to the liver mediated by high-density lipoprotein (HDL) protects against the development of atherosclerosis (3). Sphingolipid-rich microdomains in the cell membrane such as caveolae and rafts (4), various receptor complexes including class BI scavenger receptors (5, 6) and HB1/2 (7), and GPI-linked proteins (e.g., CD36) (8) have been suggested to be involved in the process of reverse cholesterol transport. Moreover, apolipoproteins, lipid transfer proteins (cholesteryl ester transfer protein, phospholipid transfer protein), and lipoprotein processing enzymes (lecithin:cholesterol acyltransferase, lipoprotein lipase, hepatic lipase) were implicated in cholesterol transport. Although several effector molecules have been proposed to participate in macrophage cholesterol efflux (6, 9), including endogenous apolipoprotein E (10) and the cholesteryl ester transfer protein (11), the detailed molecular mechanisms underlying cholesterol export in these cells have not yet been characterized.
Recently, mutations of the ATP-binding cassette (ABC) transporter ABCA1 gene have been causatively linked to familial HDL deficiency and Tangier disease (12–14). ABC transporters constitute a family of transport molecules that couple the energy of ATP hydrolysis to the translocation of substrates (allokrites) across biologic membranes (15, 16). ABCG1, a recently cloned ABC transporter (also known as ABC8 or human white gene), is a member of the subgroup of half-size transporters (17, 18). It exhibits high homology with the Drosophila white gene, which acts as a regulator of tryptophan and guanine uptake in conjunction with the scarlet and brown genes (19, 20).
In the mammalian system, no definitive function has been reported for ABCG1 to date. In the present study we identified genes that are involved in macrophage cholesterol transport and thus may be of potential relevance for the process of foam cell formation. We report here the implication of ABCG1 in the regulation of macrophage cholesterol efflux.
Materials and Methods
Cultivation of Monocytes/Macrophages.
Monocytes were obtained from healthy normolipidemic volunteers by leukapheresis and were purified by counterflow elutriation. Monocyte purity was >95% as confirmed by FACS analysis (Becton Dickinson). Aliquots of 1 × 106/ml monocytes were cultured in macrophage serum-free medium (GIBCO/BRL). After 12 h, fresh macrophage medium was added supplemented with 50 ng/ml human recombinant M-CSF (R & D Systems). This medium was used in all experiments.
Lipoproteins.
LDL (d = 1.006–1.063 g/ml) and HDL3 (d = 1.125–1.21 g/ml) were prepared from human plasma of healthy donors using standard methods. LDL was acetylated through the repeated addition of acetic anhydride followed by dialysis in PBS (21).
Determination of Cholesteryl Esters.
Cellular lipids were extracted as described (22). In brief, samples were dissolved in CHCl3/MeOH and were separated by using external standards on 10- × 20-cm silica gel HPTLC plates (Merck) in a 80:20:1 mixture of n-hexane, diethylether, and acetic acid (>99%). Plates were stained and quantified on a TLC scanner II (Camag, Berlin).
RNA Extraction and Northern Blot Analysis.
Total RNA was isolated by using the guanidine isothiocyanate method (23). The ABCG1 specific probe was generated from cDNA by using the primers 5′GATCAATCGCATTCATTTA3′ (forward) and 5′TCCTTCTTTGTTTGTTATAT3′ (reverse). Filters were rehybridized with a glyceraldehyde-3-phosphate dehydrogenase-specific probe to verify that comparable RNA amounts were loaded. Tissue-specific expression of ABCG1 was assessed by using a multiple tissue poly(A)+ RNA master blot from various human tissues (Clontech). The blots were quantitated densitometrically, and the individual signal densities were calculated relative to the mRNA expression in the liver (set to 100%).
Differential Display.
Differential display screening for genes that are regulated by cholesterol import or export was performed as follows: 0.2 μg of total RNA was reverse-transcribed by utilizing specific, anchored oligo(dT) primers (Metabion, Munich; GeneAmp RNA PCR core kit, Perkin–Elmer). Oligo(dT) primers had two additional nucleotides at their respective 3′ ends: an invariable A at the second-to-last position (3′ end) and A, C, G, or T at the last position. A 13-mer oligo(dT) (T101: 5′T11AG3′) was used in a 20-μl PCR reaction (2.5 μmol/liter). One-tenth of the reverse transcription reaction was amplified by PCR (2.5 units of Taq DNA polymerase, 1.25 mM MgCl2) using the oligo(dT) and an arbitrary upstream primer (D20 5′GATCAATCGC3′) (2.5 μmol/liter). The amplification profile was 94°C, 30 sec; 41°C, 60 sec; 72°C, 30 sec; 40 cycles. Amplification products were separated on a 10% polyacrylamide gel and were visualized by silver staining (24). cDNA fragments of interest were reamplified, blunted, and cloned into a pUC18 vector and were sequenced. These fragments were used as Northern blot probes.
Antisense Experiments.
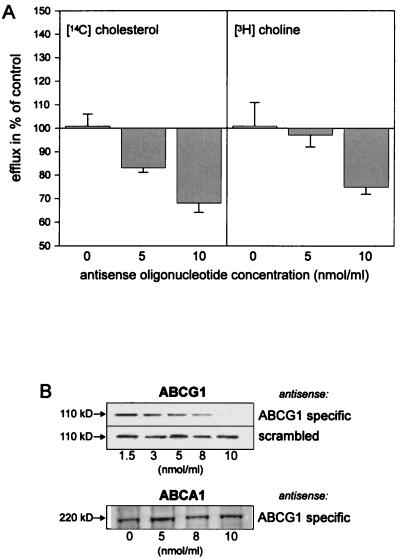
Macrophages were incubated with different ABCG1 specific antisense oligonucleotides (n = 17) (Metabion) at the concentrations 5 or 10 nmol/ml. Cytotoxic effects were monitored by fluorescence microscopy of propidium iodide stained cells. The percentage of nonviable cells was <5% for all oligonucleotide concentrations used. Among the tested antisense molecules, the oligonucleotide 5′-TGCCGACCGAGAAAG-3′ showed a concentration-dependent reduction of ABCG1 protein expression (Fig. 3). This oligonucleotide was used in the lipid efflux experiments. A scrambled oligonucleotide containing identical nucleotides served as control.
Figure 3.
(A) Inhibition of ABCG1 expression reduces cholesterol and phospholipid efflux from lipid-laden macrophages. Macrophages were incubated with 14C-cholesteryl ester and 3H-choline-labeled acLDL for 2 days in the presence of a selected antisense oligonucleotide directed against human ABCG1 or a scrambled oligonucleotide (control) at the indicated concentrations. Cells were subsequently deloaded in the presence of HDL3 for 12 h. Shown is the HDL3-mediated efflux of intracellular 14C-cholesteryl ester and 3H-choline from macrophages that were incubated in the presence of the ABCG1-specific antisense oligonucleotide. Data are indicated relative to the efflux in cells that were treated with the control antisense oligonucleotide (0 nmol, 100%) and represent two independent experiments, each measured in triplicate. The mean counts per minute in the media and cell fractions were ABCG1 specific antisense oligonucleotide: 5 nmol, medium: 34073 (chol), 99900 (pl), cells: 71178 (chol), 347829 (pl); 10 nmol, medium: 14625 (chol), 76172 (pl), cells: 42061 (chol), 350954 (pl); control oligonucleotide: 5 nmol, medium: 34342 (chol), 99264 (pl), cells: 54279 (chol), 346660 (pl), 10 nmol, medium: 26922 (chol), 106220 (pl), cells: 44708 (chol), 339588 (pl); no oligonucleotide: medium: 38196 (chol); 113005 (pl), cells: 76361 (chol); 449088 (pl). (B) Concentration-dependent suppression of ABCG1 expression in macrophages during treatment with the ABCG1-specific antisense oligonucleotide. Cells were incubated for 2 days with acLDL in the presence of the ABCG1-specific antisense (Top) or the scrambled control oligonucleotide (Middle). Note that the expression of the cholesterol responsive transporter ABCA1 is not down-regulated by the ABCG1-specific antisense oligonucleotide (Bottom).
Assessment of Cholesterol and Phosphatidylcholine Efflux after Incubation with Antisense Oligonucleotides.
To measure the efflux of cholesterol and phospholipids, macrophages were pulsed with 1.5 μCi/ml 14C-cholesterol and 3 μCi/ml 3H-choline chloride (NEN) for 2 days in the presence of acetylated LDL (acLDL) and two different concentrations of antisense oligonucleotides. Subsequently, the cells were incubated in the presence or absence of HDL3 for 12 h. The 14C-cholesterol and 3H-choline content of the media and cell lysates (total protein content: 30–50 μg) was measured, and the efflux was determined as percent of total 14C-cholesterol and 3H-choline loaded (25).
Generation of Antibodies.
A polyclonal antiserum against amino acids 616–630 of human ABCG1 (17) was generated in chicken (Pineda AK-Service, Berlin) and tested for specificity. Optimal titers for Western blot analysis and immunocytochemistry were determined.
Western Blot Analysis.
Total protein from macrophages was isolated by using standard techniques. Aliquots of 40 μg of protein were separated on a 12% SDS/PAGE and were transferred onto a PVDF membrane (Fluorotrans transfer membrane, Pall). ABCG1 was detected by using the chicken antibody (1:1,000 dilution) and a peroxidase-conjugated goat anti-chicken antibody (Sigma) (1:5,000). ABCA1 was visualized by using the polyclonal antiserum ABC1/18 (Pineda) recognizing the sequence GEESDEKSHPGS of ABCA1 (1:200).
Flow Cytometry.
To assess ABCG1 surface expression, cultured monocytes/macrophages were harvested, washed, and stained with a saturating concentration of the polyclonal ABCG1 antiserum followed by incubation with a FITC-labeled affinity-purified donkey anti-chicken F(ab)2 fragment (Jackson ImmunoResearch). For the determination of the intracellular expression of ABCG1, macrophages were fixed with 4% paraformaldehyde, were permeabilized (0.1% saponin), and were stained as described above. Preimmune chicken serum was used as an isotype control. Flow cytometric analyses were performed on a FACSCalibur flow cytometer (Becton-Dickinson).
Light Microscopic Immunocytochemistry.
Cultured cells were fixed in Zamboni's fixative and were incubated in 1% NaBH4 after a washing step. Endogenous peroxidases were inhibited by using 3% H2O2. A subset of cells was permeabilized with 0.2% Triton X-100 for intracellular ABCG1 staining. The primary ABCG1 antiserum (1:200) was used in combination with an affinity-purified peroxidase-conjugated rabbit anti-chicken antibody (1:1,000, Sigma). The reaction was developed by utilizing 3,3′-DAB as a chromogen, followed by counterstaining with hematoxylin. In control experiments, either the primary antiserum or the secondary antibody were omitted, or preimmune serum alone was used.
Semiquantitative Reverse Transcription–PCR.
The regulation of known human ABC transporter genes in macrophages in response to lipid loading and deloading was assessed by semiquantitative reverse transcription–PCR. Aliquots of 1 μg of total RNA were reverse-transcribed and PCR amplified (cycle profile: 92.5°C, 44 sec; 60°C, 40 sec; and 71.5°C, 46 sec). To monitor that PCR was in the exponential phase, each gene was amplified for 25, 30, and 35 cycles, respectively. Gene-specific primers were selected from published sequences or own cloning experiments (see details in Table 3, published as supplemental data on the PNAS web site, www.pnas.org). Amplification of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase served to normalize input RNA amounts.
Results
Kinetics of Cholesterol Loading and Deloading in Human Macrophages.
To assess the cholesterol import and export capacity in our culture model, the cellular cholesterol content of macrophages was determined during acLDL loading (100 μg/ml) and subsequent deloading in the presence of the cholesterol acceptor HDL3 (100 μg/ml; data not shown). We found that macrophages could take up cholesterol up to 120 nmol/mg cellular protein during acLDL-mediated influx of 3 days. In untreated control cells, no cholesteryl ester accumulation was observed. The cholesterol export kinetics during incubation of macrophages with HDL3 was assessed for HDL3-dependent and -independent cholesterol efflux. For this, macrophages were loaded with acLDL for 2 days in the presence of 14C-cholesterol as described (25–27). Cells were then washed and deloaded in the presence of HDL3 for 12, 24, and 48 h, respectively. Control macrophages were incubated in the absence of exogenous acLDL. After the chase period, the 14C-cholesterol content in the medium and cell fraction were determined separately. The cholesterol efflux from macrophages was significantly enhanced by HDL3 when compared with untreated control cells. The cholesterol content in macrophages was reduced to 68% after 12 h of incubation with HDL3, but only to 86% in the absence of HDL3. After 48 h, HDL3-treated cells exhibited only 50% of the amount of loaded 14C-cholesterol measured in controls. These results indicated that our lipid loading and deloading protocol provided conditions that allowed the efficient acLDL-mediated import and HDL3-mediated export of cholesterol in macrophages.
Cholesterol-Dependent Regulation of ABCG1 in Human Monocyte-Derived Macrophages.
To identify macrophage genes that are regulated by cholesterol influx and efflux, we assessed gene expression in differentiating monocytes during cholesterol loading and deloading by differential display. Freshly obtained human monocytes were loaded with acLDL (100 μg/ml) for 60 h in the presence of M-CSF, followed by 12 h of deloading using HDL3 (100 μg/ml). Total RNA was extracted for each time point and from appropriate untreated controls and was subjected to differential display analysis. Using this approach, we found that the expression of ABCG1, the human homolog of the Drosophila white gene, was induced by acLDL loading and subsequently down-regulated by incubation with HDL3. In addition, we identified the ABC transporter ABCA1 by the same approach.
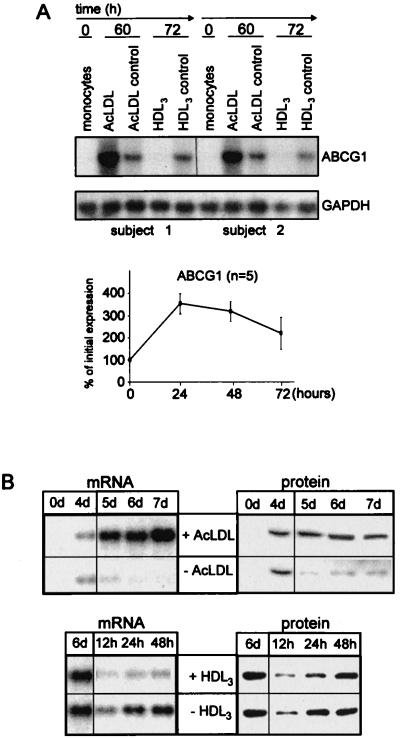
The ABCG1 gene regulation profile during cholesterol import and export, as assessed by differential display, was confirmed by Northern blot analysis using total RNA from macrophages of several different individuals. The results demonstrated that in freshly obtained monocytes cholesterol loading with acLDL strongly induces ABCG1 expression and cholesterol efflux mediated by HDL3 completely suppresses the expression of ABCG1 (Fig. 1A). AcLDL loading induced rapid ABCG1 surface expression as assessed by flow cytometry using an anti-human ABCG1antiserum (Fig. 1A). ABCG1 mRNA was not detectable in freshly isolated monocytes; however, an increase in ABCG1 expression during monocyte differentiation into macrophages was observed (Fig. 1A, acLDL control).
Figure 1.
Regulation of ABCG1 in human monocytes/macrophages during acLDL and HDL3 mediated lipid influx and efflux. (A) ABCG1 mRNA expression during loading and deloading of monocytes with acLDL (100 μg/ml) and HDL3 (100 μg/ml), respectively (shown for two representative individuals). Freshly isolated monocytes (0 h) were allowed to differentiate into macrophages for 60 h in the presence or absence of acLDL (acLDL control). Subsequently, cells were deloaded for 12 h, using HDL3 as exogenous lipid acceptor, or were maintained in culture without further intervention (HDL3 control). Expression of glyceraldehyde-3-phosphate dehydrogenase mRNA is shown as a reference. (Lower) ABCG1 surface expression during AcLDL loading of human monocytes/macrophages as assessed by FACS. (B) Regulation of ABCG1 mRNA and protein expression in differentiated human macrophages in the presence of acLDL and HDL3. (Upper) After a 4-day differentiation period (4d), macrophages were incubated with acLDL (+ acLDL) until days 5–7. Macrophages maintained in the absence of acLDL served as controls (− acLDL). 0d represents freshly isolated monocytes. (Lower) After 2 days of incubation with acLDL (6d), lipid efflux was induced by HDL3 (+ HDL) for 12, 24, and 48 h, respectively. Control cells were maintained in the absence of exogenous lipid acceptors (− HDL). A polyclonal antiserum recognizing human ABCG1 was used in the FACS and Western blot analyses.
To determine the effect of cholesterol influx and efflux on the regulation of ABCG1 in predifferentiated macrophages, monocytes were allowed to differentiate for 4 days before lipid loading and deloading. The ABCG1 mRNA regulation pattern in response to cholesterol influx and efflux in differentiated macrophages (Fig. 1B) was consistent with that observed in differentiating monocytes (Fig. 1A). Both ABCG1 mRNA and protein levels were up-regulated by cholesterol loading and were down-regulated by HDL3 in differentiated macrophages, indicating that cholesterol transport had a similar impact on ABCG1 gene and protein expression (Fig. 1B). Flow cytometric analysis in cholesterol-laden macrophages showed a 114 ± 21% (n = 3) increase in ABCG1 surface expression and a 66 ± 18% (n = 2) increase in intracellular labeling as compared with unloaded cells.
Immunocytochemical Analysis of ABCG1 Expression in Cholesterol-Laden Macrophages.
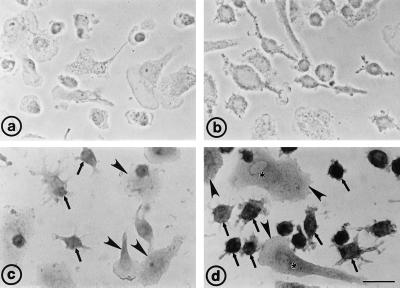
Light microscopic immunocytochemical analysis of monocytes that were cultured for 1 day showed only occasional immunolabeling (not shown). Most monocytes formed small groups, and the peripheral cells frequently exhibited long processes without specific immunostaining. After 4 days of culturing, a weak intracellular immunoreactivity for ABCG1 was detectable in almost all cells (not shown), and unequivocal signs of adherence and spreading were observed (Fig. 2a). Differentiated macrophages that were incubated with acLDL for 2 days showed ABCG1 specific intracellular staining (Fig. 2d), which was most abundant in small, rounded-off cells that lacked long processes (Fig. 2d). In contrast, spread-out macrophages displayed faint ABCG1-specific immunoreactivity, localized predominantly in the perinuclear (endoplasmic reticulum and Golgi-rich) region. Controls showed no (Fig. 2b) or weak nonspecific binding (Fig. 2c). The immunolocalization experiments, showing an increase in ABCG1 expression in cholesterol-laden macrophages, were thus consistent with the immunoblot results (Fig. 1B).
Figure 2.
Light microscopic immunocytochemical analysis of ABCG1 expression in cultured human macrophages. (a) Immunostaining of unloaded macrophages cultured for 4 days without addition of the secondary antibody (control 1). The absence of staining verifies the complete inhibition of endogenous peroxidases. (b) Macrophages incubated with acLDL for 2 days, stained without primary antiserum (control 2), indicating the complete blocking of nonspecific binding sites of the secondary antibody. (c) AcLDL-laden macrophages as in b, incubation with preimmune serum (control 3). Flattened cells (arrowheads) show less nonspecific labeling than smaller cells that extend discrete cellular processes (arrows). (d) Intracellular ABCG1 immunostaining in acLDL-laden macrophages. Note the intense staining in small macrophages (arrows) compared with large spread-out cells (arrowheads). A faint labeling in the perinuclear region (asterisks) is visible in flattened cells. Immunosignals were visualized by using the immunoperoxidase technique, and cell nuclei were counterstained with hematoxylin. (Bar = 20 μm.)
Tissue Distribution of ABCG1 mRNA.
Expression analysis in a variety of human tissues demonstrated a ubiquituous expression of ABCG1 mRNA, as observed for ABCA1 (Table 1). Unlike ABCA1 (28), ABCG1 showed highest expression in adrenal glands, lung, heart, and spleen and appeared to be less abundantly expressed in fetal tissues.
Table 1.
Expression of ABCG1 mRNA in human tissues compared to the expression of ABCA1 (28)
| Tissue | Relative ABCG1 mRNA expression, percent | Relative ABCA1 mRNA expression, percent |
|---|---|---|
| Adrenal gland | 126 | 99 |
| Thymus | 82 | 78 |
| Lung | 135 | 96 |
| Heart | 108 | 75 |
| Skeletal muscle | 54 | 51 |
| Brain | 66 | 44 |
| Spleen | 106 | 56 |
| Lymphnode | 54 | 52 |
| Pancreas | 26 | 29 |
| Placenta | 107 | 145 |
| Colon | 61 | 49 |
| Small intestine | 61 | 80 |
| Prostate | 47 | 48 |
| Testis | 23 | 49 |
| Ovary | 37 | 48 |
| Uterus | 32 | 63 |
| Mammary gland | 37 | 29 |
| Thyroid gland | 37 | 60 |
| Kidney | 75 | 26 |
| Liver | 100 | 100 |
| Bone marrow | 30 | 13 |
| Peripheral leukocytes | 26 | 56 |
| Fetal brain | 32 | 62 |
| Fetal liver | 34 | 123 |
| Fetal spleen | 74 | 89 |
| Fetal thymus | 50 | 70 |
| Fetal lung | 60 | 93 |
mRNA levels for individual tissues were assessed by dot blot analysis and were analyzed densitometrically. Expression values are indicated for each gene relative to its expression in the liver (100%).
Inhibition of ABCG1 Using Antisense Oligonucleotides Results in Reduced Cholesterol and Phospholipid Efflux from Cholesterol-Laden Macrophages.
Because ABCG1 is regulated by acLDL and HDL3, it is conceivable that ABCG1 is directly involved in the translocation of cholesterol and phospholipids. We therefore assessed cholesterol and phospholipid efflux in macrophages during selective inhibition of ABCG1 expression. During acLDL loading, macrophages were incubated with different antisense oligonucleotides targeting ABCG1 at concentrations of 5 nmol/ml and 10 nmol/ml, respectively, and subsequently incubated with HDL3 for 12 h. Although incubation with high oligonucleotide concentrations resulted in slightly diminished acLDL uptake, no significant differences were seen between antisense treated and control cells (data not shown).
A concentration-dependent reduction of ABCG1 protein expression was found for 1 of 17 tested antisense oligonucleotides (“AS”). In two independent experiments, using this oligonucleotide, a 16.8 ± 0.2% (5 nmol AS) and 32.0 ± 0.4% (10 nmol AS) overall reduction in macrophage cholesterol efflux was observed compared with cells that were treated with a scrambled control oligonucleotide (Fig. 3A). A 25 ± 0.3% reduction of phospholipid efflux occurred when macrophages were incubated with AS at a concentration of 10 nmol/ml whereas no significant effect was observed with a lower oligonucleotide concentration (Fig. 3A).
Western blot analysis showed a dose-dependent suppression of macrophage ABCG1 expression for the ABCG1-specific AS, which was almost 100% at a concentration of 10 nmol/ml. In contrast, no reduction of protein expression was observed in cells that were treated with control oligonucleotides (Fig. 3B). To validate these observations, we assessed whether the ABCG1-specific AS had a negative effect on the expression of ABCA1. This ABC transporter has recently been demonstrated to be critically involved in cellular lipid and lipoprotein metabolism (12). Moreover, it showed an identical regulatory response to acLDL loading and HDL3-mediated lipid release in macrophages (Table 2). However, no significant differences in ABCA1 expression levels were found using various concentrations of AS (Fig. 3B). Thus, our antisense experiments strongly suggest that the observed reduction of cholesterol efflux in cholesterol laden macrophages was attributable to the specific suppression of ABCG1 expression.
Table 2.
Expression and cholesterol-dependent gene regulation of ABC transporters in human macrophages
| Gene | Chromosome localization | Half-full-size* | Peripheral blood monocytes | 3-day-old macrophages (M-CSF) | Cholesterol loading (acLDL) | Cholesterol deloading (HDL3) | Known or putative molecules transported |
|---|---|---|---|---|---|---|---|
| ABCG1 (ABC8) |
21q22.3 | hs | + | ↑ | ↑↑ | ↓↓ | Cholesterol/choline PL |
| ABCA1 (ABC1) |
9q22-31 | fs | + | ↑ | ↑↑ | ↓↓ | Cholesterol/IL-1β |
| ABCC5 (MRP5) |
3q25-27 | fs | + | ↑ | ↑↑ | ↓ | Xenobiotics |
| ABCB11 (BSEP, SPGP) |
2q24 | fs | + | ↑ | ↑↑ | ↓ | Bile acids |
| ABCD1 (ALDP, ALD) |
Xq28 | hs | + | ↑ | ↑ | ↓ | Very long chain fatty acids |
| ABCA5 (EST90625) |
17q24 | fs | + | ↑ | ↑ | ↓ | |
| ABCC2 (MRP2) |
10q23-24 | fs | + | + | ↑ | ↓ | Bilirubin glucuronide glutathione conjugates |
| ABCB6‡ (EST45597) |
2q33-36 | hs | + | + | ↑ | ↓ | |
| ABCA7† (ABC4) |
19p13 | fs | + | + | ↑ | ↓ | |
| ABCC1 (MRP1) |
16p13.12 | fs | + | ↓ | ↑ | ↓ | Sphingolipids, eicosanoids |
| ABCA3 (ABC3) |
16p13.3 | fs | + | ↑ | ↑ | nr | |
| EST1133530‡ | 4p16-pter | fs | + | ↑ | ↑ | nr | |
| ABCB4 (MDR3) |
7q21 | fs | + | ↑ | ↓ | ↑ | Phosphatidylcholine |
| ABCG2 (EST157481,ABCP) |
4q22-23 | hs | + | ↑ | ↓ | ↑ | Xenobiotics |
| ABCC4 (MRP4) |
13q31 | fs? | + | ↑ | ↓ | ↑ | Xenobiotics |
| ABCB9 (EST122234) |
12q24 | hs | + | ↑ | ↓ | ↑ | |
| ABCD2 (ALDR) |
12q11 | hs | + | ↓ | ↓ | ↑ | Very long chain fatty acids |
| ABCB1 (MDR1) |
7q21 | fs | + | + | ↓ | ↑ | Phospholipids, amphiphiles |
| ABCA6 (EST155051) |
17q24 | fs | + | ↑ | ↓ | nr | |
| EST640918‡ | 17q24 | fs | + | ↑ | ↓ | nr | |
| ABCD4 (P70R) |
14q24.3 | hs | + | ↑ | nr | nr | Very long chain fatty acids |
| ABCA2 (ABC2) |
9q34 | fs | + | ↑ | nr | nr | Estramustine |
| ABCF2 (EST133090) |
7q35-36 | hs | + | ↑ | nr | nr | |
| ABCB7 (ABC7) |
Xq13.1-3 | hs | + | ↑ | nr | nr | Iron |
| ABCF1 (ABC50, TSAP) |
6q21.33 | hs | + | ↑ | nr | nr | |
| ABCC6 (MRP6) |
16p13.11 | fs | + | ↓ | nr | nr | Xenobiotics |
| ABCB5 (EST422562) |
7p14 | fs | + | ↓ | nr | nr | |
| ABCC3 (MRP3) |
17q11-21 | fs | + | nr | nr | nr | |
| ABCA4 (ABCR) |
1p22 | fs | + | nr | nr | nr | Retinoids, lipofuscin |
| ABCB2 (TAP1) |
6p21.3 | hs | + | nr | nr | nr | Peptides |
| ABCB3 (TAP2) |
6p21.3 | hs | + | nr | nr | nr | Peptides |
| ABCF3 (EST201864) |
3q25.1-2 | hs | + | nr | nr | nr | |
| ABCB8 (EST328128) |
7q35-36 | hs | + | ↑ | nr | nr | |
| ABCE1 (OABP) |
4q31 | hs | + | ↑ | nr | nr | |
| ABCB10 (EST20237) |
1q32 | hs | + | ↑ | nr | nr | |
| EST698739‡ | 17q24 | fs | + | ↑ | nr | nr | |
| ABCC10 (EST182763) |
6p21 | fs | + | nr | nr | nr | |
| ABCC7 (CFTR) |
7q31 | fs | − | − | − | − | Ions |
| ABCC8 (SUR-1) |
11p15.1 | fs | − | − | − | − | Potassium |
| ABCD3 (PMP70) |
1p21-22 | hs | − | − | − | − | Very long chain fatty acids |
| Huwhite2‡ | 11q23 | hs | − | − | − | − | |
| EST168043‡ | 2p15-16 | hs | − | − | − | − | |
| EST990006‡ | 17q24 | fs | − | − | − | − |
The new designations for ABC transporters according to the suggestions of the Human Gene Nomenclature Committee were used (old in parentheses). ↑, upregulation; ↓ downregulation; +, expressed; −, not expressed; nr, not regulated.
Half or full size transporters as deduced from the predicted amino acid sequence.
† Identical with clone DKFZp597J0214Q2 (Resource Center of the German Human Genome Project); homologous to the mouse gene presented by C. Broccardo, M.-F. Luciani, B. Jordan, and G. Chimini at the Second FEBS Advanced Lecture Course, Gosau, Austria, Feb. 20–27, 1999 (www.med.rug.nl/mdl/humanabc.htm).
‡ Information provided by R.A. and M.D.
Identification of Further Cholesterol-Responsive Members of the Family of ABC Transporter Genes.
A systematic survey of the expression of currently known human ABC genes (n = 43) revealed that 37 (86%) were expressed in human monocyte-derived macrophages. We then tested the hypothesis that other members of the ABC transporter family are regulated by cholesterol loading and deloading in human macrophages. Using our standardized semiquantitative reverse transcription–PCR approach, we identified a total of 20 ABC transporters that were regulated on the mRNA expression level during acLDL-mediated import or HDL3 mediated export of cholesterol. A detailed synopsis of the results of the gene expression screening, suggesting the potential involvement of a variety of ABC transporters in macrophage cholesterol transport, is shown in Table 2.
Discussion
Our results presented here suggest a direct involvement of ABCG1 in macrophage lipid export processes and show a function for this ABC transporter in the mammalian system. Efflux of cholesterol from cells is thought to take place in cholesterol and sphingolipid-rich membrane microdomains including rafts (29) and caveolae (2), which have been implicated in HDL-mediated cholesterol efflux (30). The integrity of rafts critically depends on their cholesterol content (31) and the maintenance of an asymmetric phospholipid microenvironment, which is critical for the physiologic activity of lipid excretory processes (32). It is possible that ABCG1 is expressed in rafts; however, detailed studies are required to determine its the exact clustering and surface localization. In addition to its surface expression, ABCG1 was detectable inside the cell, predominantly in the perinuclear compartment. Although this may be the reflection of the normal biosynthetic pathway, it is possible that ABCG1 is also involved in intracellular lipid transport processes.
We could specifically suppress the expression of ABCG1 by using oligonucleotides directed against ABCG1 mRNA. This was paralleled by a significant reduction of cholesterol and phospholipid efflux from acLDL-laden macrophages, strongly suggesting that ABCG1 is an active component of the macrophage lipid export complex. The inhibition of lipid efflux in macrophages was less pronounced than the suppression of ABCG1 expression, which was almost complete. This reflects most likely the presence of ABCG1-independent lipid efflux mechanisms such as passive aqueous diffusion processes. In addition, it is also conceivable that putative functional partners of ABCG1 partially compensate the lack of ABCG1.
The finding that ABCG1 is involved in the translocation of at least two groups of lipophilic substrates points to a relatively broad lipid translocase specificity of this transporter, as it has been reported for MDR2 (32). Moreover, the active involvement of ABCG1 in macrophage reverse cholesterol transport suggests its potential implication in the process of foam cell formation. ABCG1 may be part of a lipid transport system that counterbalances excessive influx of cholesterol into macrophages. This view is consistent with the strong induction of ABCG1 expression in response to acLDL loading. Because we found that ABCG1 is expressed ubiquitously in human tissues, its role in cellular lipid homeostasis may not be limited to macrophages.
In Drosophila, the ABCG1 homolog white gene acts as a dimeric importer for eye pigment precursors. The white gene product associates with one of the partner polypeptides brown or scarlet to form the active transporter unit. It is thus possible that human ABCG1 forms heterodimers with several heterologous partners. The recently characterized half-size transporter ABCG2, the so far only other known member of the G subfamily of ABC transporters (33), may be functionally associated with ABCG1. However, our results showing reciprocal mRNA regulation patterns for ABCG1 and ABCG2 during lipid loading and deloading do not support this notion (Table 2). Alternatively, it has been concluded by theoretical exclusion of potential dimerization partners in yeast that ADP1, the yeast homolog of ABCG1, may exist as homodimer (34). Although not likely, it is thus conceivable that ABCG1 may form homodimers.
The comprehensive search for lipid flux responsive genes among all currently known 43 human ABC transporters family identified a group of 37 transporters that are expressed in human macrophages. Among these, a total of 20 transporters were found to be regulated by lipid loading and deloading, suggesting a role for additional ABC transporters in macrophage lipid transport processes. Importantly, the group of lipid-responsive transporters includes ABCA1, which, in the course of this study, has been shown to be a major regulator of HDL metabolism and a cause of Tangier disease (12–14). Moreover, the group of cholesterol-responsive ABC genes comprises transporters that are known to translocate lipophilic substances (MRP1, MRP2, MRP5, BSEP) and two transporters, MDR1 and MDR3, for which phospholipid transport activity has been shown (35–37) (Table 2). An involvement of these ABC transporters in macrophage lipid transport has not yet been reported (38). Genetic alterations in some of the lipid flux responsive transporters have been associated with other human diseases. These include mutations of the MRP2, MDR3, and BSEP genes that have been reported to account for the Dubin-Johnson syndrome (39) and subforms of progressive familial intrahepatic cholestasis (35, 40). Furthermore, mutations of the ALDP gene lead to the neurodegenerative disorder adrenoleukodystrophy, which is associated with impaired peroxisomal β-oxidation of very long chain fatty acids (41).
In light of these data, it can be expected that additional members of the group of cholesterol-responsive ABC transporters may be involved in cellular lipid transport and processes that are critical for the maintenance of cell membrane integrity. Efforts are underway to characterize the detailed role of ABCG1 and other lipid flux-responsive ABC transporters in macrophage lipid homeostasis. This may potentially lead to the development of novel antiatherogenic intervention strategies based on the understanding of the role of macrophage ABC transporters.
Supplementary Material
Acknowledgments
We are grateful to Ulrike Stöckl, Renate Glätzl, and Stella Potra for expert technical assistance. This work has been supported by a grant from BAYER AG Pharma-Research, Wuppertal, Germany (Dr. K. D. Bremm).
Abbreviations
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- ABC
ATP-binding cassette
- acLDL
acetylated LDL
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ross R. Nature (London) 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Steinbrecher U P. Biochim Biophys Acta. 1999;1436:279–298. doi: 10.1016/s0005-2760(98)00127-1. [DOI] [PubMed] [Google Scholar]
- 3.Tall A R. Eur Heart J. 1998;19, Suppl. A:A31–A35. [PubMed] [Google Scholar]
- 4.Fielding C J, Fielding P E. J Lipid Res. 1997;38:1503–1521. [PubMed] [Google Scholar]
- 5.Ji Y, Jian B, Wang N, Sun Y, Moya M L, Phillips M C, Rothblat G H, Swaney J B, Tall A R. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Doi T, Hamakubo T, Kodama T. Cell Mol Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tozuka M, Fidge N. Biochem J. 1989;261:239–244. doi: 10.1042/bj2610239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisanti M P, Scherer P E, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu Y H, Cook R F, Sargiacomo M. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz G, Orso E, Rothe G, Klucken J. Curr Opin Lipidol. 1997;8:287–300. doi: 10.1097/00041433-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Stohr J, Schindler G, Rothe G, Schmitz G. Arterioscler Thromb Vasc Biol. 1998;18:1424–1432. doi: 10.1161/01.atv.18.9.1424. [DOI] [PubMed] [Google Scholar]
- 11.Ishigami M, Yamashita S, Sakai N, Hirano K I, Arai T, Maruyama T, Takami S, Koyama M, Kameda-Takemura K, Matsuzawa Y. Eur J Clin Invest. 1997;27:285–292. doi: 10.1046/j.1365-2362.1997.1040657.x. [DOI] [PubMed] [Google Scholar]
- 12.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, et al. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 13.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette J C, Deleuze J F, Brewer H B, Duverger N, Denefle P, Assmann G. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 14.Brooks-Wilson A, Marcil M, Clee S M, Zhang L H, Roomp K, van Dam M, Yu L, Brewer C, Collins J A, Molhuizen H O, et al. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 15.Hyde S C, Emsley P, Hartshorn M J, Mimmack M M, Gileadi U, Pearce S R, Gallagher M P, Gill D R, Hubbard R E, Higgins C F. Nature (London) 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 16.Dean M, Allikmets R. Curr Opin Genet Dev. 1995;5:779–785. doi: 10.1016/0959-437x(95)80011-s. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Rossier C, Lalioti M D, Lynn A, Chakravarti A, Perrin G, Antonarakis S E. Am J Hum Genet. 1996;59:66–75. [PMC free article] [PubMed] [Google Scholar]
- 18.Jack R S, Grunwald U, Stelter F, Workalemahu G, Schutt C. Eur J Immunol. 1995;25:1436–1441. doi: 10.1002/eji.1830250545. [DOI] [PubMed] [Google Scholar]
- 19.Tearle R G, Belote J M, McKeown M, Baker B S, Howells A J. Genetics. 1989;122:595–606. doi: 10.1093/genetics/122.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreesen T D, Johnson D H, Henikoff S. Mol Cell Biol. 1988;8:5206–5215. doi: 10.1128/mcb.8.12.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein J L, Ho Y K, Basu S K, Brown M S. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz G, Lenczyk M, Ord D, Bowyer D E, Assmann G. J Chromatogr. 1984;307:81–89. doi: 10.1016/s0378-4347(00)84074-8. [DOI] [PubMed] [Google Scholar]
- 23.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 24.Lohmann J, Schickle H, Bosch T C. BioTechniques. 1995;18:200–202. [PubMed] [Google Scholar]
- 25.Rogler G, Trumbach B, Klima B, Lackner K J, Schmitz G. Arterioscler Thromb Vasc Biol. 1995;15:683–690. doi: 10.1161/01.atv.15.5.683. [DOI] [PubMed] [Google Scholar]
- 26.Drobnik W, Mollers C, Resink T, Schmitz G. Arterioscler Thromb Vasc Biol. 1995;15:1369–1377. doi: 10.1161/01.atv.15.9.1369. [DOI] [PubMed] [Google Scholar]
- 27.Mollers C, Drobnik W, Resink T, Schmitz G. Cell Signalling. 1995;7:695–707. doi: 10.1016/0898-6568(95)00041-m. [DOI] [PubMed] [Google Scholar]
- 28.Langmann T, Klucken J, Reil M, Liebisch G, Luciani M F, Chimini G, Kaminski W E, Schmitz G. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 29.Harder T, Simons K. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 30.Nion S, Briand O, Lestavel S, Torpier G, Nazih F, Delbart C, Fruchart J C, Clavey V. Biochem J. 1997;328:415–423. doi: 10.1042/bj3280415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel S N, Manthey C L, Perera P Y, Li Z Y, Henricson B E. Prog Clin Biol Res. 1995;392:421–431. [PubMed] [Google Scholar]
- 32.Elferink R P, Tytgat G N, Groen A K. FASEB J. 1997;11:19–28. doi: 10.1096/fasebj.11.1.9034162. [DOI] [PubMed] [Google Scholar]
- 33.Allikmets R, Schriml L M, Hutchinson A, Romano-Spica V, Dean M. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 34.West M A, Seatter S C, Bellingham J, Clair L. Surgery. 1995;118:220–228. doi: 10.1016/s0039-6060(05)80327-7. [DOI] [PubMed] [Google Scholar]
- 35.de Vree J M, Jacquemin E, Sturm E, Cresteil D, Bosma P J, Aten J, Deleuze J F, Desrochers M, Burdelski M, Bernard O, et al. Proc Natl Acad Sci USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith A J, Timmermans-Hereijgers J L, Roelofsen B, Wirtz K W, van Blitterswijk W J, Smit J J, Schinkel A H, Borst P. FEBS Lett. 1994;354:263–266. doi: 10.1016/0014-5793(94)01135-4. [DOI] [PubMed] [Google Scholar]
- 37.van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, van Meer G. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 38.Deleuze J F, Jacquemin E, Dubuisson C, Cresteil D, Dumont M, Erlinger S, Bernard O, Hadchouel M. Hepatology. 1996;23:904–908. doi: 10.1002/hep.510230435. [DOI] [PubMed] [Google Scholar]
- 39.Kartenbeck J, Leuschner U, Mayer R, Keppler D. Hepatology. 1996;23:1061–1066. doi: 10.1053/jhep.1996.v23.pm0008621134. [DOI] [PubMed] [Google Scholar]
- 40.Strautnieks S S, Bull L N, Knisely A S, Kocoshis S A, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, et al. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 41.Aubourg P, Mosser J, Douar A M, Sarde C O, Lopez J, Mandel J L. Biochimie. 1993;75:293–302. doi: 10.1016/0300-9084(93)90089-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.