Abstract
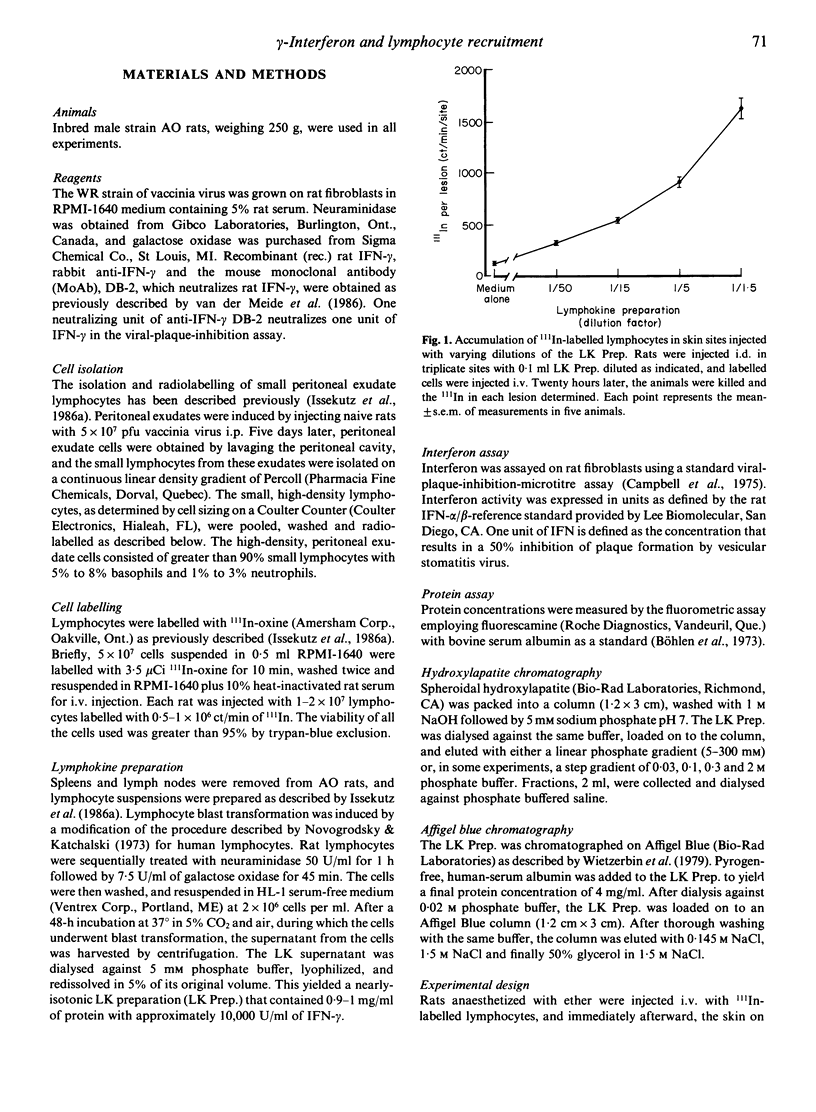
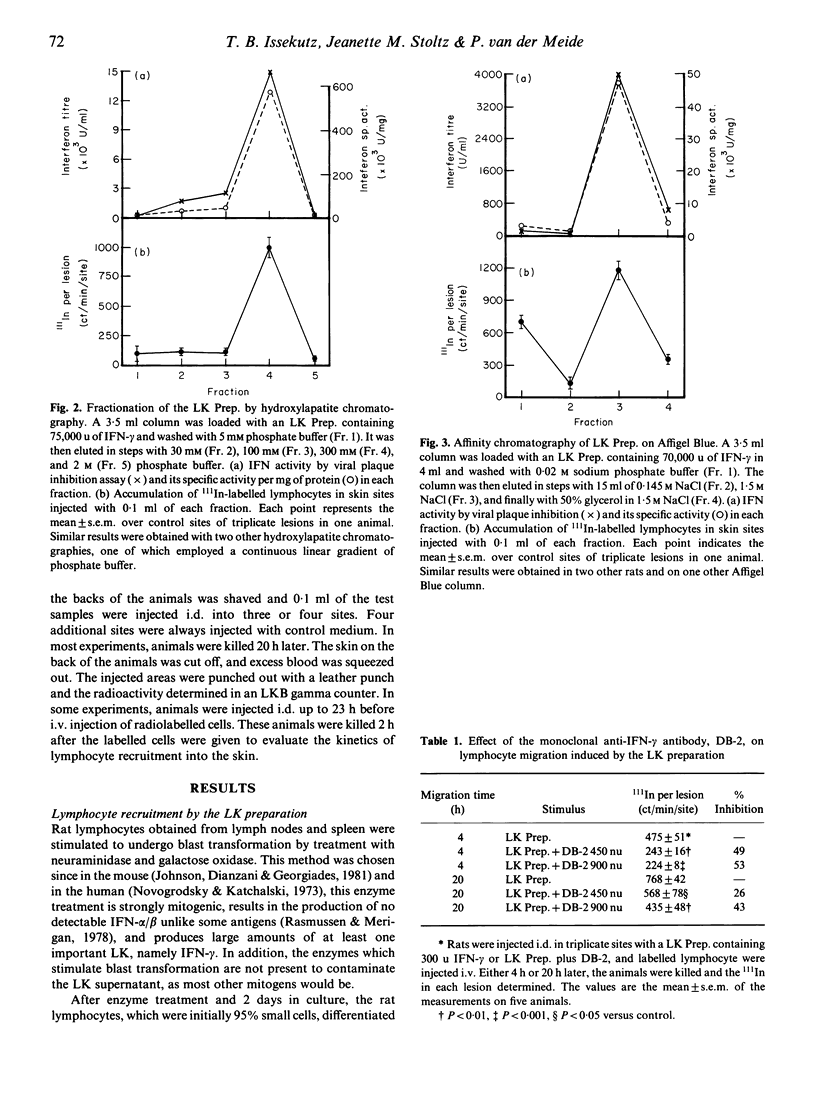
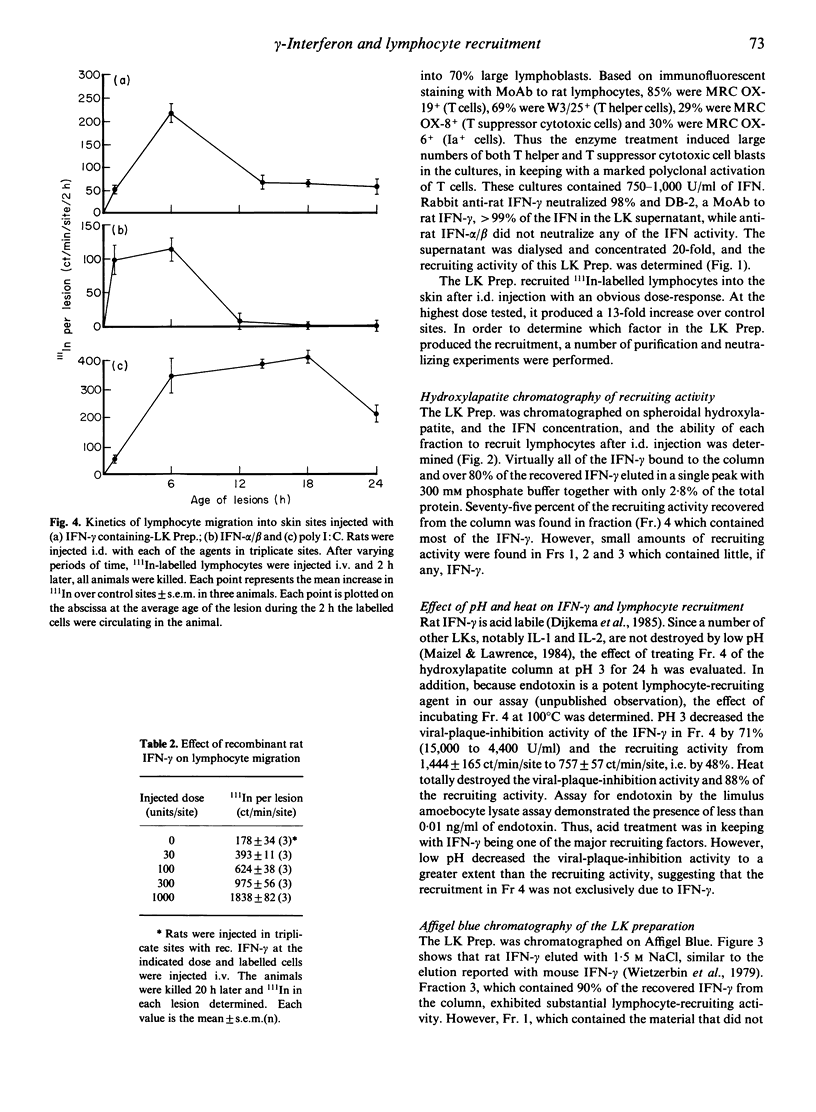
Numerous lymphocytes are recruited from the blood into cutaneous DTH reactions. Alpha/beta-interferon (IFN) and its inducers can recruit lymphocytes into the skin after i.d. injection, but activated T lymphocytes, which are responsible for DTH, produce very little IFN-alpha/beta. Our goal was to determine the major T cell lymphokine (LK) which could stimulate the migration of lymphocytes into the skin. Rats were injected i.d. with LK containing supernatants from activated T cells, and lymphocyte recruitment was measured by the accumulation of 111In-labelled lymphocytes in the skin. Large numbers of labelled cells migrated into sites injected with the LKs. The major portion of the recruiting activity of the LKs coeluted with IFN-gamma after hydroxylapatite and Affigel Blue chromatography, although a second recruiting factor was also found. Both the recruiting and IFN anti-viral activities were partially destroyed by pH 3. A monoclonal anti-IFN-gamma antibody inhibited up to 53% of the recruitment observed after 4 h and up to 43% after 20 h. Kinetic studies showed that maximal recruitment occurred 6 h after i.d. injection of the LKs. Recombinant rat IFN-gamma also stimulated lymphocyte migration into the skin. Histologically, sites injected with IFN-gamma showed a mononuclear cell infiltrate. It is suggested that IFN-gamma is the major mediator of lymphocyte recruitment produced by activated T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher E. C., Scollay R. G., Weissman I. L. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980 Jul;10(7):556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Campbell J. B., Grunberger T., Kochman M. A., White S. L. A microplaque reduction assay for human and mouse interferon. Can J Microbiol. 1975 Aug;21(8):1247–1253. doi: 10.1139/m75-186. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkema R., van der Meide P. H., Pouwels P. H., Caspers M., Dubbeld M., Schellekens H. Cloning and expression of the chromosomal immune interferon gene of the rat. EMBO J. 1985 Mar;4(3):761–767. doi: 10.1002/j.1460-2075.1985.tb03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn A. M., Schreiber A. B., Butcher E. C. Interferon-gamma regulates an antigen specific for endothelial cells involved in lymphocyte traffic. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9114–9118. doi: 10.1073/pnas.83.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J., Jones C. J., Bromley M., Andrews P. Changes in vascular endothelium related to lymphocyte collections in diseased synovia. Arthritis Rheum. 1983 Dec;26(12):1427–1433. doi: 10.1002/art.1780261203. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Harris P. C., Yamamoto R. S., Crane J., Granger G. A. The human LT serum. X. The initial form released by T-enriched lymphocytes is 150,000 m.w., associated with small nonlytic components, and can dissociate into the smaller alpha, beta, and gamma m.w. classes. J Immunol. 1981 Jun;126(6):2165–2170. [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., Webster D. M. Role of interferon in lymphocyte recruitment into the skin. Cell Immunol. 1986 May;99(2):322–333. doi: 10.1016/0008-8749(86)90241-8. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Webster D. M., Stoltz J. M. Lymphocyte recruitment in vaccinia virus-induced cutaneous delayed-type hypersensitivity. Immunology. 1986 May;58(1):87–94. [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Dianzani F., Georgiades J. A. Large-scale induction and production of human and mouse immune interferons. Methods Enzymol. 1981;78(Pt A):158–162. doi: 10.1016/0076-6879(81)78111-4. [DOI] [PubMed] [Google Scholar]
- MCCLUSKEY R. T., BENACERRAF B., MCCLUSKEY J. W. STUDIES ON THE SPECIFICITY OF THE CELLULAR INFILTRATE IN DELAYED HYPERSENSITIVITY REACTIONS. J Immunol. 1963 Mar;90:466–477. [PubMed] [Google Scholar]
- Maizel A. L., Lachman L. B. Control of human lymphocyte proliferation by soluble factors. Lab Invest. 1984 Apr;50(4):369–377. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Induction of lymphocyte transformation by sequential treatment with neuraminidase and galactose oxidase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1824–1827. doi: 10.1073/pnas.70.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Grant B. W., Eddy A. A., Michael A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983 Oct 1;158(4):1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Cotran R. S., Gitlin J. D., Fiers W., Clayberger C., Krensky A. M., Burakoff S. J., Reiss C. S. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983 Oct 20;305(5936):726–729. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Merigan T. C. Role of T lymphocytes in cellular immune responses during herpes simplex virus infection in humans. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3957–3961. doi: 10.1073/pnas.75.8.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietzerbin J., Stefanos S., Lucero M., Falcoff E., O'Malley J. A., Sulkowski E. Physico-chemical characterization and partial purification of mouse immune interferon. J Gen Virol. 1979 Sep;44(3):773–781. doi: 10.1099/0022-1317-44-3-773. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]


