Abstract
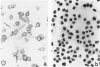
Anti-nuclear antibodies (ANA) in serum were detected in male ICR mice fed drinking water containing 3, 30 and 300 ppm Cd as CdCl2 for 10 weeks. In response to Cd exposure, ICR mice developed ANA of the IgG class giving nuclear patterns moderately stained by immunofluorescence or immunoenzyme method. Positive immunofluorescence staining of ANA was obtained in 50, 89 and 90% of ICR mice exposed to 3, 30 and 300 ppm Cd, respectively. Their spleen cells also showed an enhancement of antibody forming response to sheep red blood cells (SRBC) without the SRBC priming. When mice were primed with SRBC after exposure to Cd, however, a significant suppression of the antibody forming response was observed in mice fed 300 ppm Cd but not in those fed 3 ppm Cd. No significant differences in delayed-type hypersensitivity reaction to SRBC were observed between Cd-fed and control animals. Inbred BALB/c mice were less susceptible to the induction of ANA by Cd, as induced only in 300 ppm Cd-fed mice. Thus environmental exposure to Cd can induce ANA in ICR mice with a high susceptibility, presumably accompanied with a non-specific stimulation of antibody formation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Segovia D. Drug-induced antinuclear antibodies and lupus syndromes. Drugs. 1976;12(1):69–77. doi: 10.2165/00003495-197612010-00003. [DOI] [PubMed] [Google Scholar]
- Bernard A., Lauwerys R., Gengoux P., Mahieu P., Foidart J. M., Druet P., Weening J. J. Anti-laminin antibodies in Sprague-Dawley and brown Norway rats chronically exposed to cadmium. Toxicology. 1984 Jun;31(3-4):307–313. doi: 10.1016/0300-483x(84)90111-2. [DOI] [PubMed] [Google Scholar]
- Castano P. Chronic intoxication by cadmium experimentally induced in rabbits. A study of kidney ultrastructure. Pathol Microbiol (Basel) 1971;37(4):280–301. doi: 10.1159/000162329. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Druet E., Sapin C., Günther E., Feingold N., Druet P. Mercuric chloride-induced anti-glomerular basement membrane antibodies in the rat: genetic control. Eur J Immunol. 1977 Jun;7(6):348–351. doi: 10.1002/eji.1830070605. [DOI] [PubMed] [Google Scholar]
- Druet P., Druet E., Potdevin F., Sapin C. Immune type glomerulonephritis induced by HgCl2 in the Brown Norway rat. Ann Immunol (Paris) 1978 Oct-Dec;129 100(6):777–792. [PubMed] [Google Scholar]
- Fujimaki H., Murakami M., Kubota K. In vitro evaluation of cadmium-induced augmentation of the antibody response. Toxicol Appl Pharmacol. 1982 Feb;62(2):288–293. doi: 10.1016/0041-008x(82)90127-2. [DOI] [PubMed] [Google Scholar]
- Hang L., Slack J. H., Amundson C., Izui S., Theofilopoulos A. N., Dixon F. J. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med. 1983 Mar 1;157(3):874–883. doi: 10.1084/jem.157.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Joshi B. C., Dwivedi C., Powell A., Holscher M. Immune complex nephritis in rats induced by long-term oral exposure to cadmium. J Comp Pathol. 1981 Jan;91(1):11–15. doi: 10.1016/0021-9975(81)90040-2. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Exon J. H., Roan J. G. Antibody suppression by cadmium. Arch Environ Health. 1975 Dec;30(12):598–601. doi: 10.1080/00039896.1975.10666787. [DOI] [PubMed] [Google Scholar]
- Koller L. D. Immunosuppression produced by lead, cadmium, and mercury. Am J Vet Res. 1973 Nov;34(11):1457–1458. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavé I., de Ruffino D. T. Altered immune response during cadmium administration in mice. Toxicol Appl Pharmacol. 1984 Jun 15;74(1):46–56. doi: 10.1016/0041-008x(84)90268-0. [DOI] [PubMed] [Google Scholar]
- Müller S., Gillert K. E., Krause C., Jautzke G., Gross U., Diamantstein T. Effects of cadmium on the immune system of mice. Experientia. 1979 Jul 15;35(7):909–910. doi: 10.1007/BF01955143. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Pierce G. B., Jr Enzyme-labeled antibodies for the light and electron microscopic localization of tissue antigens. J Cell Biol. 1967 May;33(2):307–318. doi: 10.1083/jcb.33.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa M., Masuko-Sato K., Takahashi K., Otsuka F. Strain differences in cadmium-mediated suppression of lymphocyte proliferation in mice. Toxicol Appl Pharmacol. 1986 Jun 30;84(2):379–388. doi: 10.1016/0041-008x(86)90146-8. [DOI] [PubMed] [Google Scholar]
- Papoian R., Pillarisetty R., Talal N. Immunological regulation of spontaneous antibodies to DNA and RNA. II. Sequential switch from IgM to IgG in NZB/NZW F1 mice. Immunology. 1977 Jan;32(1):75–79. [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Hirsch F., Sapin C., Druet P. Autoreactive T cells in mercury-induced autoimmune disease: in vitro demonstration. J Immunol. 1986 Oct 15;137(8):2548–2554. [PubMed] [Google Scholar]
- Robinson C. J., Abraham A. A., Balazs T. Induction of anti-nuclear antibodies by mercuric chloride in mice. Clin Exp Immunol. 1984 Nov;58(2):300–306. [PMC free article] [PubMed] [Google Scholar]
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Shenker B. J., Matarazzo W. J., Hirsch R. L., Gray I. Trace metal modification of immunocompetence. I. Effect of trace metals in the cultures on in vitro transformation of B lymphocytes. Cell Immunol. 1977 Nov;34(1):19–24. doi: 10.1016/0008-8749(77)90225-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. T., Ratajczak H. V., Aranyi C., Gibbons R., Fenters J. D. Evaluation of host resistance and immune function in cadmium-exposed mice. Toxicol Appl Pharmacol. 1985 Sep 30;80(3):446–456. doi: 10.1016/0041-008x(85)90389-8. [DOI] [PubMed] [Google Scholar]
- Weening J. J., Fleuren G. J., Hoedemaeker P. J. Demonstration of antinuclear antibodies in mercuric chloride-induced glomerulopathy in the rat. Lab Invest. 1978 Oct;39(4):405–411. [PubMed] [Google Scholar]
- Weening J. J., Grond J., van der Top D., Hoedemaeker P. J. Identification of the nuclear antigen involved in mercury-induced glomerulopathy in the rat. Invest Cell Pathol. 1980 Apr-Jun;3(2):129–134. [PubMed] [Google Scholar]
- Weening J. J., Hoedemaeker P. J., Bakker W. W. Immunoregulation and anti-nuclear antibodies in mercury-induced glomerulopathy in the rat. Clin Exp Immunol. 1981 Jul;45(1):64–71. [PMC free article] [PubMed] [Google Scholar]