Abstract
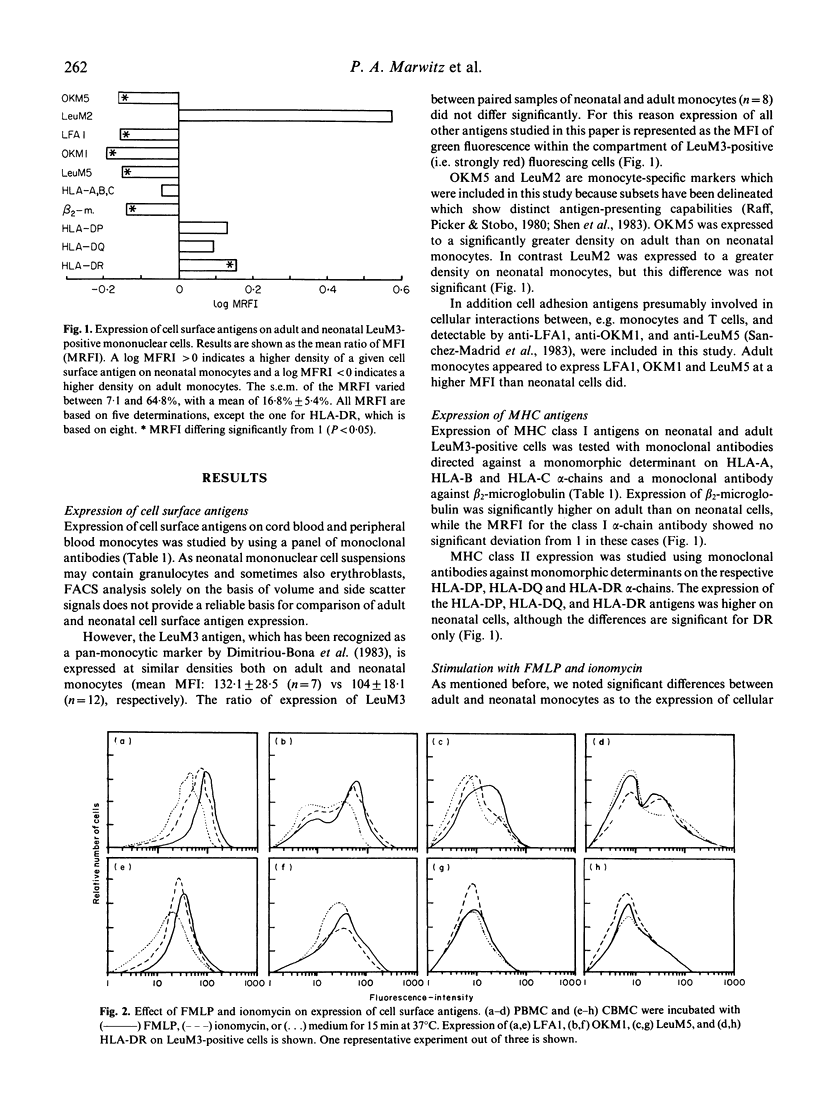
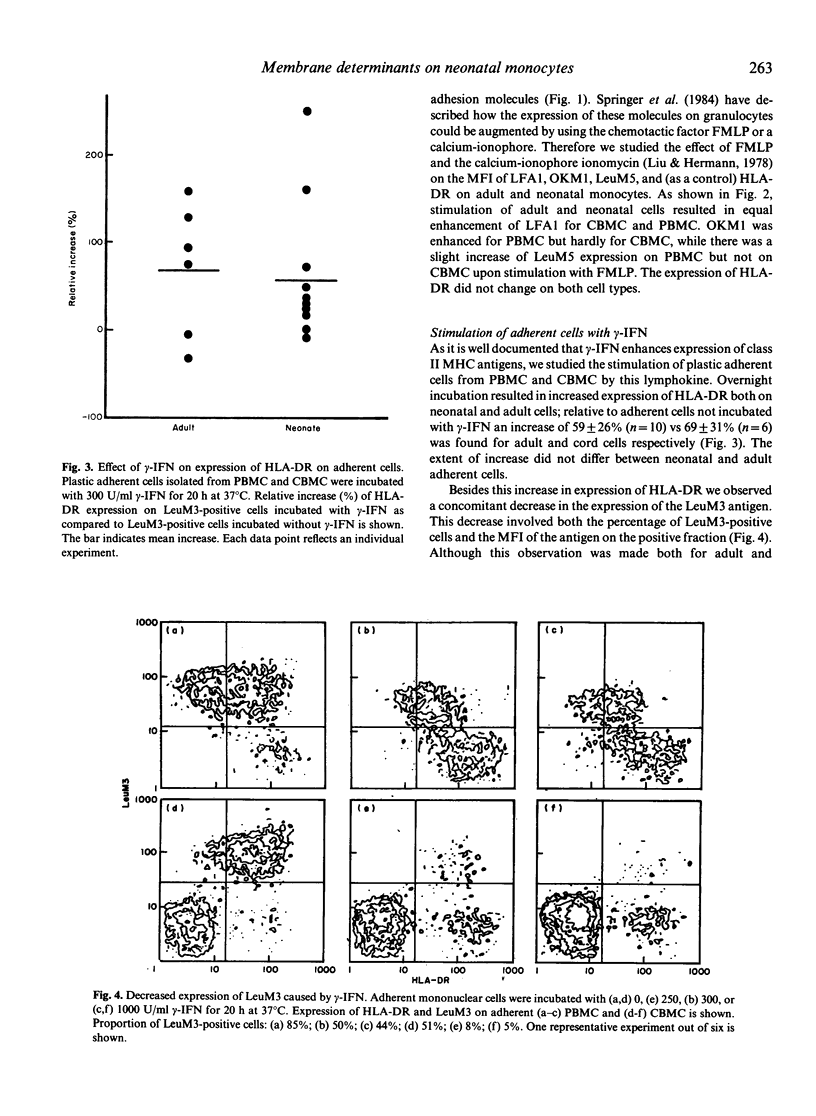
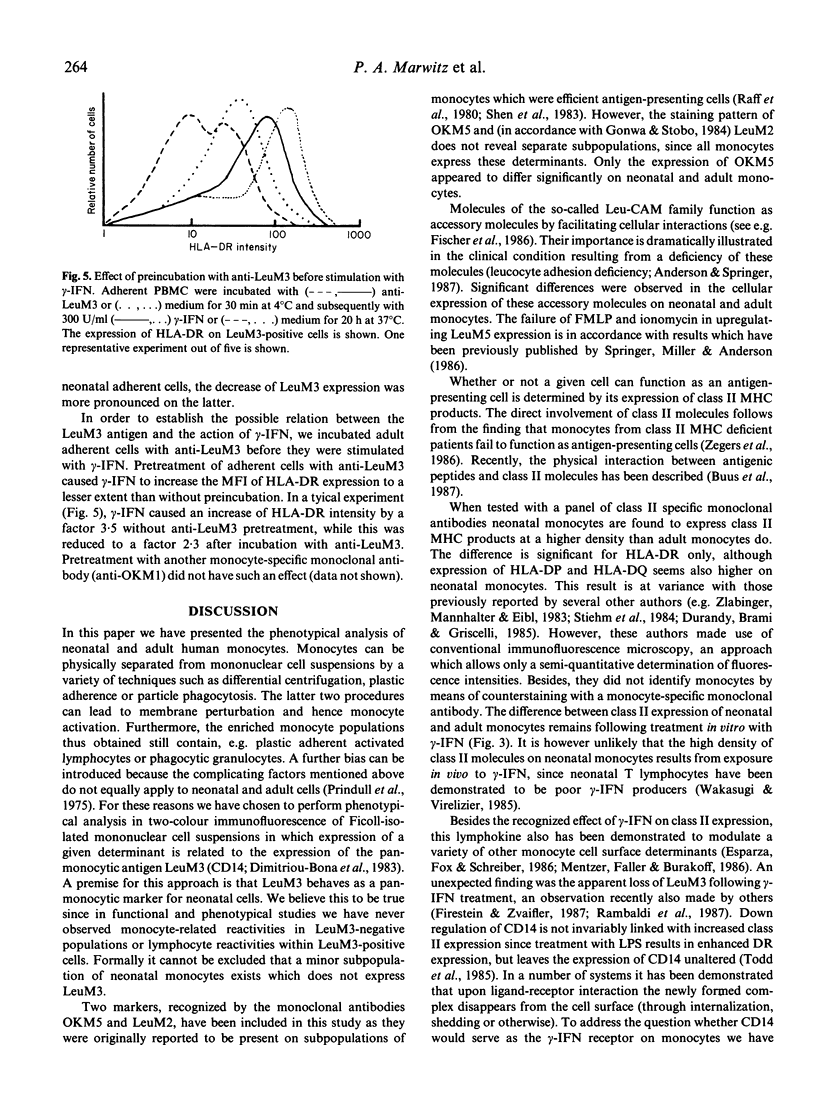
The difference between newborn and adult mononuclear cells in the antigen dose required for optimal antibody production in vitro can be ascribed to differences in the antigen-presenting capacities of the respective monocytes (Van Tol et al., 1984b). We have therefore studied the expression of cell surface determinants on human neonatal and adult monocytes by the use of monoclonal antibodies to membrane proteins including MHC antigens. No difference was observed in the expression of LeuM3 with regard to both the percentage of positive cells and the density of the respective determinant. In contrast, neonatal cells express the antigens OKM5, LFA1, OKM1 and LeuM5 at a lower density than adult cells do. The same holds for beta 2-microglobulin, but neonatal and adult monocytes express MHC class I alpha-chains at a similar density, whereas among the class II MHC antigens, HLA-DR is significantly more highly expressed on neonatal cells. This difference remains after treatment in vitro with gamma-interferon (gamma-IFN). Treatment with gamma-IFN also resulted in a less dense expression of the LeuM3 antigen. Preincubation of monocytes with LeuM3 monoclonal antibody partially abrogates subsequent upregulation of class II MHC antigens by gamma-IFN, a phenomenon observed with both neonatal and adult monocytes. These data indicate a functional involvement of LeuM3 with the cellular action of gamma-IFN. Taken together, the cell surface phenotype of neonatal monocytes is that of a highly efficient antigen presenting cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Durandy A., Brami C., Griscelli C. The effects of indomethacin administration during pregnancy on women's and newborns' T-suppressor lymphocyte activity and on HLA class II expression by newborns' leukocytes. Am J Reprod Immunol Microbiol. 1985 Jul;8(3):94–100. doi: 10.1111/j.1600-0897.1985.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Esparza I., Fox R. I., Schreiber R. D. Interferon-gamma-dependent modulation of C3b receptors (CR1) on human peripheral blood monocytes. J Immunol. 1986 Feb 15;136(4):1360–1365. [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Down regulation of human monocyte differentiation antigens by interferon gamma. Cell Immunol. 1987 Feb;104(2):343–354. doi: 10.1016/0008-8749(87)90036-0. [DOI] [PubMed] [Google Scholar]
- Fischer A., Durandy A., Sterkers G., Griscelli C. Role of the LFA-1 molecule in cellular interactions required for antibody production in humans. J Immunol. 1986 May 1;136(9):3198–3203. [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Taniguchi T. Intracytoplasmic phosphorylation sites of Tac antigen (p55) are not essential for the conformation, function, and regulation of the human interleukin 2 receptor. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9650–9654. doi: 10.1073/pnas.83.24.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman S. J., Faltynek C. R., Baglioni C. Binding of human recombinant 125I-interferon gamma to receptors on human cells. J Biol Chem. 1985 Jan 25;260(2):1191–1195. [PubMed] [Google Scholar]
- Liu C., Hermann T. E. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978 Sep 10;253(17):5892–5894. [PubMed] [Google Scholar]
- Mentzer S. J., Faller D. V., Burakoff S. J. Interferon-gamma induction of LFA-1-mediated homotypic adhesion of human monocytes. J Immunol. 1986 Jul 1;137(1):108–113. [PubMed] [Google Scholar]
- Orchansky P., Rubinstein M., Fischer D. G. The interferon-gamma receptor in human monocytes is different from the one in nonhematopoietic cells. J Immunol. 1986 Jan;136(1):169–173. [PubMed] [Google Scholar]
- Pabst H. F., Kreth H. W. Ontogeny of the immune response as a basis of childhood disease. J Pediatr. 1980 Oct;97(4):519–534. doi: 10.1016/s0022-3476(80)80003-5. [DOI] [PubMed] [Google Scholar]
- Prindull G., Prindull B., Palti Z., Yoffey J. M. Phagocytic cells in cord blood. Biol Neonate. 1975;27(5-6):318–328. doi: 10.1159/000240789. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Picker L. J., Stobo J. D. Macrophage heterogeneity in man. A subpopulation of HLA-DR-bearing macrophages required for antigen-induced T cell activation also contains stimulators for autologous-reactive T cells. J Exp Med. 1980 Sep 1;152(3):581–593. doi: 10.1084/jem.152.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A., Young D. C., Herrmann F., Cannistra S. A., Griffin J. D. Interferon-gamma induces expression of the interleukin 2 receptor gene in human monocytes. Eur J Immunol. 1987 Jan;17(1):153–156. doi: 10.1002/eji.1830170127. [DOI] [PubMed] [Google Scholar]
- Rebaï N., Malissen B. Structural and genetic analyses of HLA class I molecules using monoclonal xenoantibodies. Tissue Antigens. 1983 Aug;22(2):107–117. doi: 10.1111/j.1399-0039.1983.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. H., Talle M. A., Goldstein G., Chess L. Functional subsets of human monocytes defined by monoclonal antibodies: a distinct subset of monocytes contains the cells capable of inducing the autologous mixed lymphocyte culture. J Immunol. 1983 Feb;130(2):698–705. [PubMed] [Google Scholar]
- Spits H., Borst J., Giphart M., Coligan J., Terhorst C., De Vries J. E. HLA-DC antigens can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984 Apr;14(4):299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehm E. R., Sztein M. B., Steeg P. S., Mann D., Newland C., Blaese M., Oppenheim J. J. Deficient DR antigen expression on human cord blood monocytes: reversal with lymphokines. Clin Immunol Immunopathol. 1984 Mar;30(3):430–436. doi: 10.1016/0090-1229(84)90028-x. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Alvarez P. A., Brott D. A., Liu D. Y. Bacterial lipopolysaccharide, phorbol myristate acetate, and muramyl dipeptide stimulate the expression of a human monocyte surface antigen, Mo3e. J Immunol. 1985 Dec;135(6):3869–3877. [PubMed] [Google Scholar]
- Van Tol M. J., Zijlstra J., Heijnen C. J., Kuis W., Zegers B. J., Ballieux R. E. Antigen-specific plaque-forming cell response of human cord blood lymphocytes after in vitro stimulation by T cell-dependent antigens. Eur J Immunol. 1983 May;13(5):390–397. doi: 10.1002/eji.1830130508. [DOI] [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J Immunol. 1985 Jan;134(1):167–171. [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- Zlabinger G. J., Mannhalter J. W., Eibl M. M. Cord blood macrophages present bacterial antigen (Escherichia coli) to paternal T cells. Clin Immunol Immunopathol. 1983 Sep;28(3):405–412. doi: 10.1016/0090-1229(83)90107-1. [DOI] [PubMed] [Google Scholar]
- van Tol M. J., Zijlstra J., Zegers B. J., Ballieux R. E. Antigen-induced plaque-forming cell responses in cultures of peripheral blood mononuclear cells of human neonates and infants. J Pediatr. 1984 Nov;105(5):738–744. doi: 10.1016/s0022-3476(84)80293-0. [DOI] [PubMed] [Google Scholar]


