Abstract
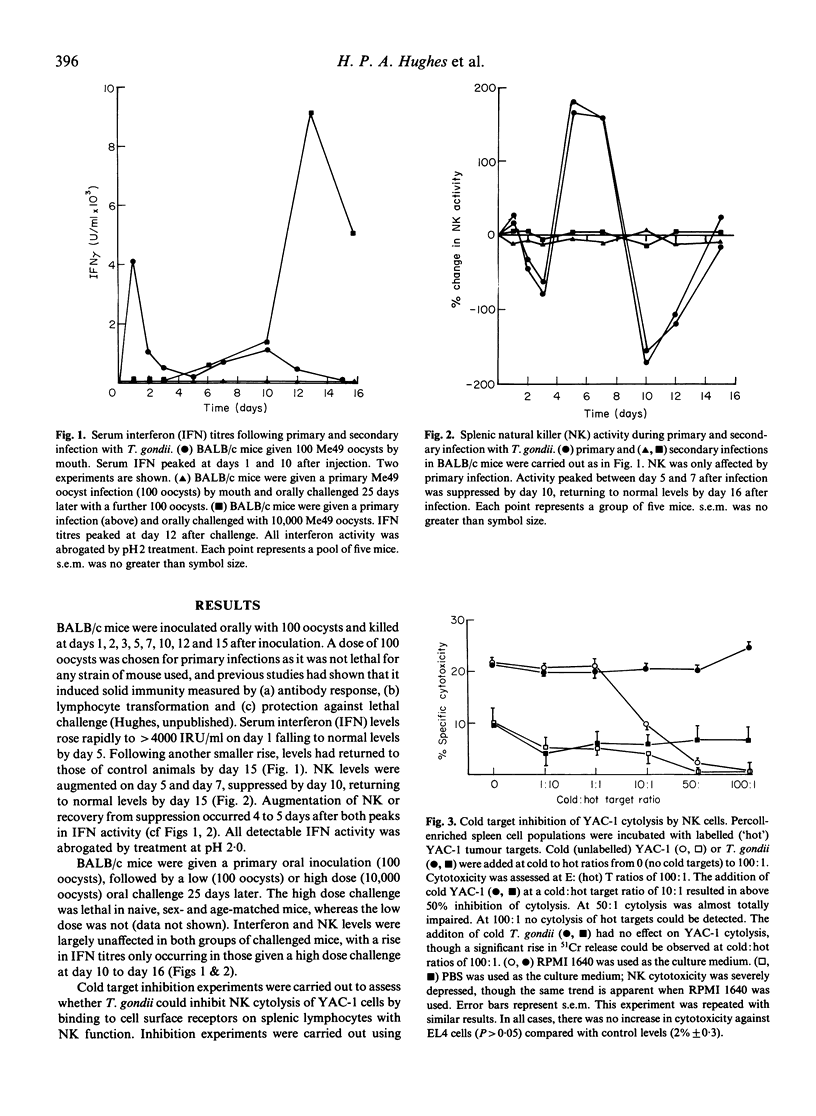
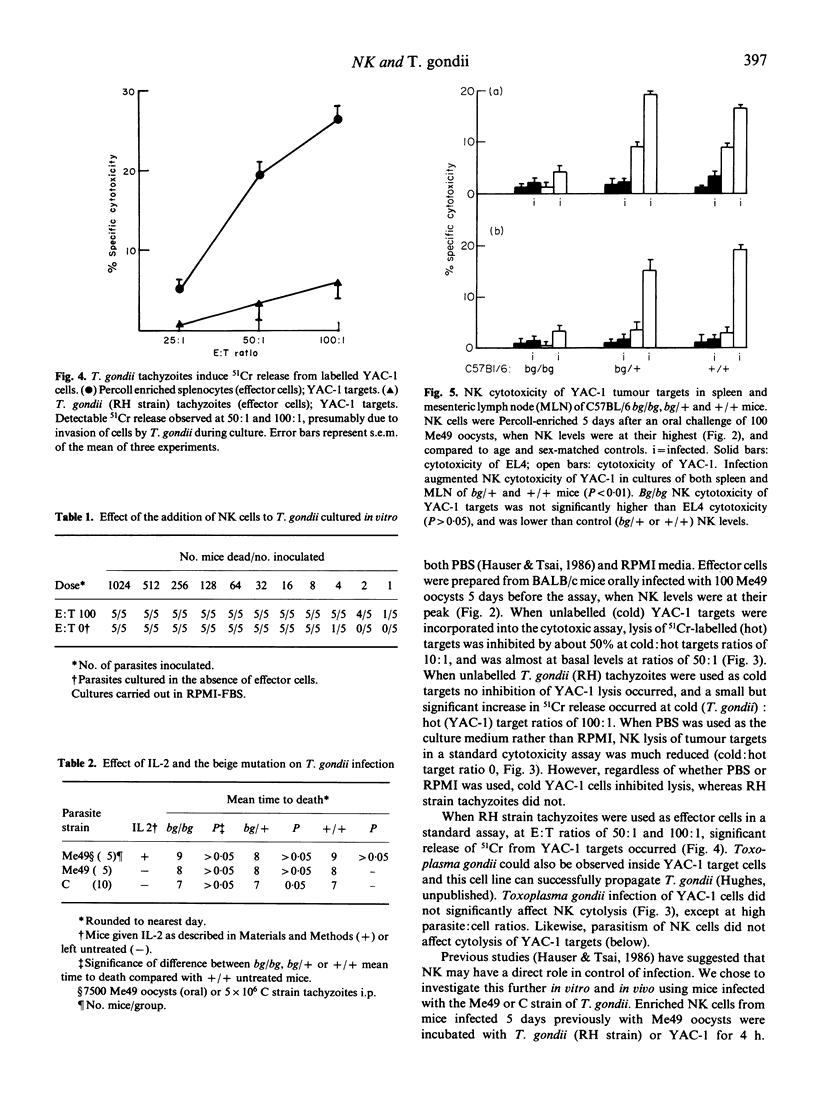
The active phase of primary and challenge oral infections of Toxoplasma gondii was investigated with respect to natural killer (NK) activity against YAC-1 tumour cell targets in vitro and serum interferon (IFN) titres. Primary (non-lethal) oral infection of BALB/c mice with Me49 oocysts resulted in a rapid increase of serum IFN titres, followed by augmented NK activity. NK levels became depressed, rising again by 15 days after infection to normal levels, again preceded by elevated IFN titres. In challenge infections NK was not augmented and IFN titres rose only if a high dose of oocysts was given. IFN activity was pH2-labile in all cases and considered to be due to IFN-gamma. Cold target inhibition studies indicated that T. gondii did not bind to NK cells. A bioassay for the effects of NK cells on T. gondii tachyzoites was developed and there was no evidence of killing in vitro by cells with NK function; T. gondii survived better when cultured with NK cells than when cultured alone. Studies using C57BL/6bg/bg,bg/+ and +/+ mice showed that there was no difference in mean time to death after administration of a lethal ME49 oocyst infection by mouth. Cytotoxicity against YAC-1 in both spleen and mesenteric lymph node (MLN) cell populations was highly augmented in bg/+ and +/+, but not in bg/bg mice. Genetic deficiency of NK activity had no effect on survival of mice after infection. Therefore NK has at best a minimal role to play in protection during the acute phase of Toxoplasma infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks C. G., Flannery G. R. Quantitative studies of natural immunity to solid tumours in rats. Persistence of natural immunity throughout reproductive life, and absence of suppressor cells in infant rats. Immunology. 1980 Feb;39(2):187–194. [PMC free article] [PubMed] [Google Scholar]
- Cerretti D. P., McKereghan K., Larsen A., Cosman D., Gillis S., Baker P. E. Cloning, sequence, and expression of bovine interferon-gamma. J Immunol. 1986 Jun 15;136(12):4561–4564. [PubMed] [Google Scholar]
- Christie E., Pappas P. W., Dubey J. P. Ultrastructure of excystment of Toxoplasma gondii oocysts. J Protozool. 1978 Nov;25(4):438–443. doi: 10.1111/j.1550-7408.1978.tb04166.x. [DOI] [PubMed] [Google Scholar]
- Georgiades J. A., Johnson H. M. Partial purification and characterization of mouse immune interferon. Methods Enzymol. 1981;78(Pt A):545–552. doi: 10.1016/0076-6879(81)78167-9. [DOI] [PubMed] [Google Scholar]
- Handman E., Chester P. M., Remington J. S. Delayed hypersensitivity to Toxoplasma and unrelated antigens in Toxoplasma-infected mice: induction and elicitation of delayed-type hypersensitivity by antigen-pulsed macrophages. Infect Immun. 1980 May;28(2):524–531. doi: 10.1128/iai.28.2.524-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Augmentation of NK cell activity by soluble and particulate fractions of Toxoplasma gondii. J Immunol. 1983 Jul;131(1):458–463. [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982 May 15;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Tsai V. Acute toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J Immunol. 1986 Jan;136(1):313–319. [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Henney C. S., Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983 Jan;130(1):222–227. [PubMed] [Google Scholar]
- Hughes H. P., Connelly C. A., Strangeways J. E., Hudson L. Antigen specific lymphocyte transformation induced by secreted antigens from Toxoplasma gondii. Clin Exp Immunol. 1984 Dec;58(3):539–547. [PMC free article] [PubMed] [Google Scholar]
- Hughes H. P., Hudson L., Fleck D. G. In vitro culture of Toxoplasma gondii in primary and established cell lines. Int J Parasitol. 1986 Aug;16(4):317–322. doi: 10.1016/0020-7519(86)90109-8. [DOI] [PubMed] [Google Scholar]
- Hughes H. P. Toxoplasmosis: the need for improved diagnostic techniques and accurate risk assessment. Curr Top Microbiol Immunol. 1985;120:105–139. doi: 10.1007/978-3-662-09197-5_6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kamiyama T., Hagiwara T. Augmented followed by suppressed levels of natural cell-mediated cytotoxicity in mice infected with Toxoplasma gondii. Infect Immun. 1982 May;36(2):628–636. doi: 10.1128/iai.36.2.628-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L. H., Currie K. M., Bradley M. S. An unexpected response to vaccination with a purified major membrane tachyzoite antigen (P30) of Toxoplasma gondii. J Immunol. 1985 May;134(5):3426–3431. [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Splenic natural killer-cell activity in mice infected with Leishmania donovani. Cell Immunol. 1984 Apr 15;85(1):201–214. doi: 10.1016/0008-8749(84)90290-9. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P., Warner J. F., Denner G. Participation of natural killer cells in the recovery of mice from visceral leishmaniasis. Cell Immunol. 1985 Apr 15;92(1):163–171. doi: 10.1016/0008-8749(85)90074-7. [DOI] [PubMed] [Google Scholar]
- Merino F., Cruz I. Natural killer activity in experimental cutaneous leishmaniasis. Int Arch Allergy Appl Immunol. 1984;73(4):347–351. doi: 10.1159/000233496. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Tait R. C., MacKenzie S., Davies M. D., Parrott D. M. Analysis of natural killer effector and suppressor activity by intraepithelial lymphocytes from mouse small intestine. Clin Exp Immunol. 1983 Apr;52(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Omata Y., Sakurai H., Izumo M., Saito A., Suzuki N. Interferon production in non-immune and immune mice after Toxoplasma infection. Nihon Juigaku Zasshi. 1984 Apr;46(2):155–165. doi: 10.1292/jvms1939.46.155. [DOI] [PubMed] [Google Scholar]
- Quan P. C., Rager-Zisman B., Wittner M., Tanowitz H. B. Interferon and natural killer cells in murine Chagas' disease. J Parasitol. 1983 Dec;69(6):1164–1166. [PubMed] [Google Scholar]
- Riccardi C., Vose B. M., Herberman R. B. Modulation of IL 2-dependent growth of mouse NK cells by interferon and T lymphocytes. J Immunol. 1983 Jan;130(1):228–232. [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]


